当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amine Chemistry of Porous CO2 Adsorbents
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2023-09-20 , DOI: 10.1021/acs.accounts.3c00367
Thien S Nguyen 1 , Nesibe A Dogan 2 , Haeseong Lim 3 , Cafer T Yavuz 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2023-09-20 , DOI: 10.1021/acs.accounts.3c00367
Thien S Nguyen 1 , Nesibe A Dogan 2 , Haeseong Lim 3 , Cafer T Yavuz 1
Affiliation
|
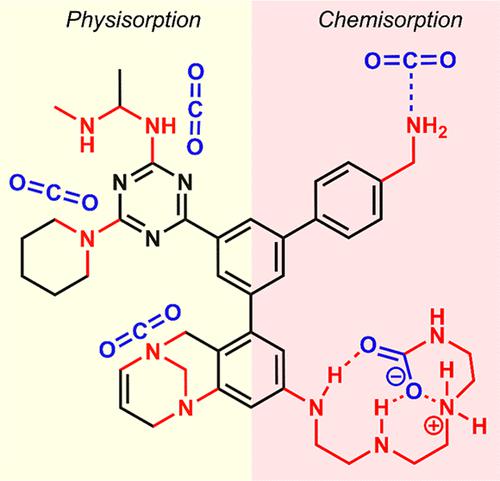
As renewable energy and CO2 utilization technologies progress to make a more significant contribution to global emissions reduction, carbon capture remains a critical component of the mission. Current CO2 capture technologies involve operations at point sources such as fossil fuel-based power plants or source-agnostic like in direct air capture. Each strategy has its own advantages and limitations, but in common, they all employ sorption-based methods with the use of sorbents strongly adhering to CO2. Amine solutions are the most widely used absorbents for industrial operations due to the robust chemical bonds formed between amines and CO2 under both dry and humid conditions, rendering excellent selectivity. Such strong binding, however, causes problematic regeneration. In contrast, purely physisorptive porous materials with high surface areas allow for the confinement of CO2 inside narrow pores/channels and have a lower regeneration energy demand but with decreased selectivity and capacity. The most promising solution would then be the unification of both types of sorbents in one system, which could bring about a practical adsorption–desorption process. In other words, the development of porous solid materials with tunable amine content is necessary to leverage the high contact surface of porous sorbents with the added ability to manipulate amine incorporation toward lower CO2 binding strength.
中文翻译:

多孔 CO2 吸附剂的胺化学
随着可再生能源和CO 2利用技术的进步,为全球减排做出更重大的贡献,碳捕获仍然是这一使命的关键组成部分。当前的CO 2捕获技术涉及点源操作,例如基于化石燃料的发电厂或不可知源(例如直接空气捕获)。每种策略都有其自身的优点和局限性,但共同点是,它们都采用基于吸附的方法,并使用与CO 2牢固粘附的吸附剂。胺溶液是工业操作中使用最广泛的吸收剂,因为胺和CO 2在干燥和潮湿条件下都能形成牢固的化学键,从而具有出色的选择性。然而,如此强的结合会导致再生问题。相反,具有高表面积的纯物理吸附多孔材料允许将CO 2限制在狭窄的孔/通道内,并且具有较低的再生能量需求,但选择性和容量降低。最有希望的解决方案是将两种类型的吸附剂统一在一个系统中,这可以带来实用的吸附-解吸过程。换句话说,开发具有可调胺含量的多孔固体材料对于利用多孔吸附剂的高接触表面以及控制胺掺入以降低CO 2 结合强度的附加能力是必要的。
更新日期:2023-09-20
中文翻译:

多孔 CO2 吸附剂的胺化学
随着可再生能源和CO 2利用技术的进步,为全球减排做出更重大的贡献,碳捕获仍然是这一使命的关键组成部分。当前的CO 2捕获技术涉及点源操作,例如基于化石燃料的发电厂或不可知源(例如直接空气捕获)。每种策略都有其自身的优点和局限性,但共同点是,它们都采用基于吸附的方法,并使用与CO 2牢固粘附的吸附剂。胺溶液是工业操作中使用最广泛的吸收剂,因为胺和CO 2在干燥和潮湿条件下都能形成牢固的化学键,从而具有出色的选择性。然而,如此强的结合会导致再生问题。相反,具有高表面积的纯物理吸附多孔材料允许将CO 2限制在狭窄的孔/通道内,并且具有较低的再生能量需求,但选择性和容量降低。最有希望的解决方案是将两种类型的吸附剂统一在一个系统中,这可以带来实用的吸附-解吸过程。换句话说,开发具有可调胺含量的多孔固体材料对于利用多孔吸附剂的高接触表面以及控制胺掺入以降低CO 2 结合强度的附加能力是必要的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号