Journal of the Taiwan Institute of Chemical Engineers ( IF 5.5 ) Pub Date : 2023-09-19 , DOI: 10.1016/j.jtice.2023.105127 M. Amirian Chegeni , Majid Rezaeivala , Saeid Karimi , Avni Berisha
|
Background
Corrosion of steel occurs due to the rough working environment in which it is used. It accelerates the deterioration of the steel components and results in substantial financial loss. Continuous research has been carried out to prevent or reduce the corrosion of steel and other metals because these materials have so many industrial applications.
Methods
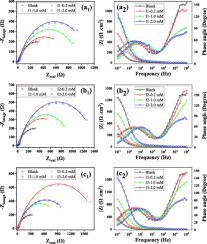
The inhibition performance of three new organic substances, N1-(3-morpholinopropyl)-N1-((pyridine-2-yl)methyl)propane-1,3-diamine (I1), 2‑methoxy-6-(((3-((3-morpholinopropyl)(pyridin-2-ylmethyl)amino)propyl)imino)methyl)phenol (I2), and its reduced form, 2‑methoxy-6-(((3-((3-morpholinopropyl)(pyridin-2-ylmethyl)amino)propyl)imino) methyl) phenol (I3), on corrosion of the P460N in 3.5 wt.% NaCl solution was investigated by potentiodynamic polarization (PDP), EIS, immersion tests, and SEM techniques.
Significant findings
A novel material exhibiting superior corrosion protection capabilities for steel was synthesized, and its efficacy was validated through both electrochemical and non-electrochemical testing methods. Comparing the OCP values in the blank and inhibitor-containing solutions shows a negative shift. According to PDP experiments, the highest value of icorr occurs with the P460N electrode in the inhibitor-free solution (79.4 μA.cm−2). Meanwhile, in the case of the highest concentration of I1, I2, and I3 inhibitors, a significant reduction in the corrosion rate was observed, with reductions of 6, 17, and 22 times, respectively. Notably, the reduced form (I3) exhibited exceptional protection against corrosion. PDP results confirmed that the inhibition efficiency reached the highest value of 84.1, 94.3, and 95.5% for I1, I2, and I3 at 2.0 mM concentration. From the EIS measurements, the inhibition performance of inhibitors follows the order I3 > I2 > I1 at equal concentration. Based on the immersion test results and SEM-EDS evaluation, the reduced sample (I3) demonstrated significantly lower corrosion over an extended period, indicating excellent long-term stability in the 3.5% NaCl solution. The electrochemical (PDP and EIS), and non-electrochemical (immersion tests) data confirmed that the inhibition efficiency of the inhibitor of I3 was better than the two other compounds. SEM and EDS analysis showed severe corrosion for the P460N surface without an inhibitor, and corrosion products such as iron oxide and trapped NaCl in porosity appeared very uneven, with different shapes. The study explored four absorption mechanisms: Langmuir, Temkin, Freundlich, and Frumkin. The three new inhibitors were found to adhere to the Langmuir adsorption isotherm with a high correlation coefficient of approximately 0.999. Moreover, these inhibitors demonstrated mixed inhibition behavior for P460N steel in a 3.5% NaCl solution.
中文翻译:

3.5 重量 P460N 钢中三种新型吗啉基抑制剂的电化学研究 % 氯化钠溶液
背景
钢材因使用环境恶劣而发生腐蚀。它加速了钢部件的劣化并导致巨大的经济损失。由于钢和其他金属具有如此多的工业应用,因此人们不断进行研究以防止或减少它们的腐蚀。
方法
三种新型有机物质N1-(3-吗啉代丙基)-N1-((吡啶-2-基)甲基)丙烷-1,3-二胺(I1)、2-甲氧基-6-(((3 -((3-吗啉代丙基)(吡啶-2-基甲基)氨基)丙基)亚氨基)甲基)苯酚(I2),及其还原形式,2-甲氧基-6-(((3-((3-吗啉代丙基))(通过动电位极化 (PDP)、EIS、浸泡测试和 SEM 技术研究了吡啶-2-基甲基)氨基)丙基)甲基)苯酚 (I3) 对 P460N 在 3.5 wt.% NaCl 溶液中的腐蚀情况。
重大发现
合成了一种对钢表现出优异的腐蚀防护能力的新型材料,并通过电化学和非电化学测试方法验证了其功效。比较空白溶液和含有抑制剂的溶液中的 OCP 值显示出负变化。根据PDP实验,在无抑制剂溶液中,i corr的最高值出现在P460N电极上(79.4 μA.cm -2)。同时,在I1、I2和I3抑制剂浓度最高的情况下,观察到腐蚀速率显着降低,分别降低了6、17和22倍。值得注意的是,还原形式 (I3) 表现出出色的防腐蚀能力。PDP结果证实,在2.0 mM浓度下,I1、I2和I3的抑制效率达到最高值,分别为84.1、94.3和95.5%。从 EIS 测量结果来看,同等浓度下抑制剂的抑制性能遵循 I3 > I2 > I1 的顺序。根据浸泡测试结果和 SEM-EDS 评估,还原样品 (I3) 在较长时间内表现出显着较低的腐蚀,表明在 3.5% NaCl 溶液中具有出色的长期稳定性。电化学(PDP 和 EIS)、和非电化学(浸泡测试)数据证实I3抑制剂的抑制效率优于其他两种化合物。SEM和EDS分析表明,在没有抑制剂的情况下,P460N表面腐蚀严重,孔隙中的氧化铁和截留的氯化钠等腐蚀产物显得非常不均匀,形状各异。该研究探索了四种吸收机制:Langmuir、Temkin、Freundlich 和 Frumkin。发现这三种新抑制剂遵循 Langmuir 吸附等温线,相关系数约为 0.999。此外,这些抑制剂在 3.5% NaCl 溶液中对 P460N 钢表现出混合抑制行为。SEM和EDS分析表明,在没有抑制剂的情况下,P460N表面腐蚀严重,孔隙中的氧化铁和截留的氯化钠等腐蚀产物显得非常不均匀,形状各异。该研究探索了四种吸收机制:Langmuir、Temkin、Freundlich 和 Frumkin。发现这三种新抑制剂遵循 Langmuir 吸附等温线,相关系数约为 0.999。此外,这些抑制剂在 3.5% NaCl 溶液中对 P460N 钢表现出混合抑制行为。SEM和EDS分析表明,在没有抑制剂的情况下,P460N表面腐蚀严重,孔隙中的氧化铁和截留的氯化钠等腐蚀产物显得非常不均匀,形状各异。该研究探索了四种吸收机制:Langmuir、Temkin、Freundlich 和 Frumkin。发现这三种新抑制剂遵循 Langmuir 吸附等温线,相关系数约为 0.999。此外,这些抑制剂在 3.5% NaCl 溶液中对 P460N 钢表现出混合抑制行为。发现这三种新抑制剂遵循 Langmuir 吸附等温线,相关系数约为 0.999。此外,这些抑制剂在 3.5% NaCl 溶液中对 P460N 钢表现出混合抑制行为。发现这三种新抑制剂遵循 Langmuir 吸附等温线,相关系数约为 0.999。此外,这些抑制剂在 3.5% NaCl 溶液中对 P460N 钢表现出混合抑制行为。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号