Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2023-09-19 , DOI: 10.1016/j.molstruc.2023.136674 Xiaochen Han , Chongyang Guo , Yugao Wang , Gang Liu , Jun Shen
|
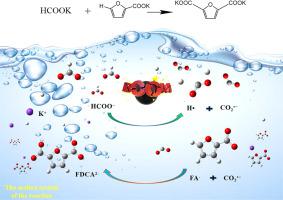
Furan-2,5-dicarboxylic acid (FDCA) is an important biomass-derived diacid, and it is the most promising substitute for terephthalic acid. The common method of FDCA usually involves 5-hydroxymethylfurfural oxidation. Edible fructose is often used as the feedstock of 5-hydroxymethylfurfural, and the oxidation step would generate undesirable impurities. Herein, FDCA was novelty synthesized by carboxylation of formate and 2-furoic acid (FA) derived from unedible-biomass. This route is potentially advantageous because it is simple to operate and does not require any additional additives. The reaction mechanism has been elucidated by density functional theory (DFT) calculations. In the carboxylation process, the C(sp2)-H bond of formate (HCOOM) undergoes cleavage, generating the carbon dioxide radical anion (CO2•−). Subsequently, CO2•− attacks the C5 of FA, forming the C C bond and ultimately yielding FDCA. The conversion of HCOOM to CO2•− is an effective way to achieve carboxylation reaction. The rate-determining step of the carboxylation reaction is the cleavage of the C(sp2)-H bond of HCOO− with activation barrier of 159.11 kJ⋅mol−1. The investigation would provide a new strategy for the mechanism of carboxylation reactions, and hold the potential to establish pathways for FDCA production.
C bond and ultimately yielding FDCA. The conversion of HCOOM to CO2•− is an effective way to achieve carboxylation reaction. The rate-determining step of the carboxylation reaction is the cleavage of the C(sp2)-H bond of HCOO− with activation barrier of 159.11 kJ⋅mol−1. The investigation would provide a new strategy for the mechanism of carboxylation reactions, and hold the potential to establish pathways for FDCA production.
中文翻译:

合成2,5-呋喃二甲的新路线:2-糠酸与甲酸酯的直接羧化
呋喃-2,5-二甲酸(FDCA)是一种重要的生物质二酸,是对苯二甲酸最有前途的替代品。FDCA的常用方法通常涉及5-羟甲基糠醛氧化。食用果糖常被用作5-羟甲基糠醛的原料,氧化步骤会产生不良杂质。在此,FDCA 是通过来自非食用生物质的甲酸盐和 2-糠酸 (FA) 的羧化而新颖合成的。该路线具有潜在的优势,因为它操作简单并且不需要任何额外的添加剂。密度泛函理论(DFT)计算阐明了反应机理。在羧化过程中,甲酸盐(HCOOM)的C(sp 2 )-H键发生裂解,产生二氧化碳自由基阴离子(CO2 •− )。随后,CO 2 •−攻击FA 的C 5,形成C  C 键并最终产生FDCA。HCOOM转化为CO 2 •−是实现羧化反应的有效途径。羧化反应的限速步骤是HCOO -的 C(sp 2 )-H 键的断裂,活化势垒为 159.11 kJ⋅mol -1。该研究将为羧化反应机制提供新策略,并有可能建立 FDCA 生产途径。
C 键并最终产生FDCA。HCOOM转化为CO 2 •−是实现羧化反应的有效途径。羧化反应的限速步骤是HCOO -的 C(sp 2 )-H 键的断裂,活化势垒为 159.11 kJ⋅mol -1。该研究将为羧化反应机制提供新策略,并有可能建立 FDCA 生产途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号