当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
BiBr3-Mediated Intramolecular Aza-Prins Cyclization of Aza-Achmatowicz Rearrangement Products: Asymmetric Total Synthesis of Suaveoline and Sarpagine Alkaloids
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-09-19 , DOI: 10.1002/anie.202311671 Wai Fung Cheng 1 , Shiqiang Ma 1 , Yin Tung Lai 1 , Yuen Tsz Cheung 1 , Kornkamon Akkarasereenon 1 , Yiqin Zhou 1 , Rongbiao Tong 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-09-19 , DOI: 10.1002/anie.202311671 Wai Fung Cheng 1 , Shiqiang Ma 1 , Yin Tung Lai 1 , Yuen Tsz Cheung 1 , Kornkamon Akkarasereenon 1 , Yiqin Zhou 1 , Rongbiao Tong 1
Affiliation
|
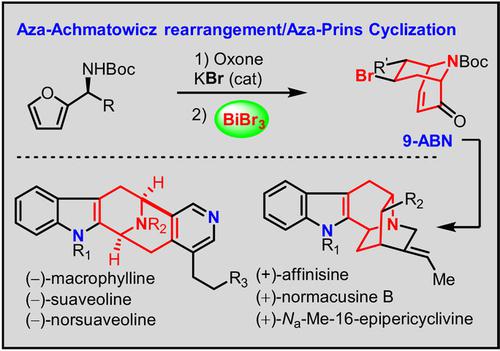
An intramolecular Prins cyclization of aza-Achmatowicz rearrangement products was developed to construct the versatile 9-azabicyclo[3.3.1]nonane (9-ABN) ring system with a variety of substitution patterns and then applied to the asymmetric total synthesis of six suaveoline and sarpagine alkaloids: macrophylline, suaveoline, norsuaveoline, affinisine, normacusine B and Na-Me-16-epipericyclivine.
中文翻译:

BiBr3 介导的 Aza-Achmatowicz 重排产物的分子内 Aza-Prins 环化:Suaveoline 和 Sarpagine 生物碱的不对称全合成
开发了 aza-Achmatowicz 重排产物的分子内 Prins 环化,以构建具有多种取代模式的通用 9-氮杂双环[3.3.1]壬烷 (9-ABN) 环系统,然后应用于六种 Suaveoline 和沙帕津生物碱:大茶碱、甜甜碱、去苏甜碱、阿菲尼辛、正马库辛 B 和N a -Me-16-表哌环利文。
更新日期:2023-09-19
中文翻译:

BiBr3 介导的 Aza-Achmatowicz 重排产物的分子内 Aza-Prins 环化:Suaveoline 和 Sarpagine 生物碱的不对称全合成
开发了 aza-Achmatowicz 重排产物的分子内 Prins 环化,以构建具有多种取代模式的通用 9-氮杂双环[3.3.1]壬烷 (9-ABN) 环系统,然后应用于六种 Suaveoline 和沙帕津生物碱:大茶碱、甜甜碱、去苏甜碱、阿菲尼辛、正马库辛 B 和N a -Me-16-表哌环利文。































 京公网安备 11010802027423号
京公网安备 11010802027423号