当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Acid-Mediated Cascade Cyclization Pathway to Indeno[2,1-c]chromen-6(7H)-ones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-09-18 , DOI: 10.1021/acs.joc.3c01459 Chander Shekhar 1 , Gedu Satyanarayana 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-09-18 , DOI: 10.1021/acs.joc.3c01459 Chander Shekhar 1 , Gedu Satyanarayana 1
Affiliation
|
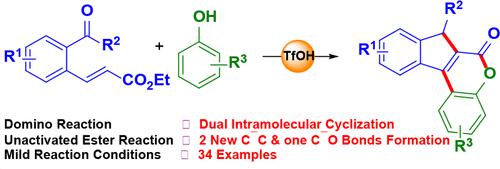
Developing mild and effective synthetic strategies for producing significant molecules starting from readily available starting materials is indispensable in organic synthesis. Herein, we present a triflic acid-driven dual cyclization pathway to produce functionalized indeno[2,1-c]chromen-6(7H)-ones from simple 2-formyl (or 2-acyl) cinnamate esters and phenols. Notably, this protocol enabled the construction of two C–C bonds and one C–O bond under metal-free reaction conditions via the activation of the unreactive ester moiety in a single pot. The isolation of intermediate indenol-ester might suggest self-intramolecular cycloaddition by the proximate double bond of the enoate ester with the o-carbonyl moiety, followed by an electrophilic attack with phenol and a subsequent cyclocondensation pathway. In addition, the photophysical properties have also been examined.
中文翻译:

酸介导的茚并[2,1-c]色烯-6(7H)-酮的级联环化途径
开发温和有效的合成策略,从容易获得的起始材料开始生产重要的分子,在有机合成中是必不可少的。在此,我们提出了一种三氟甲磺酸驱动的双环化途径,从简单的2-甲酰基(或2-酰基)肉桂酸酯和酚类生产功能化的茚并[2,1- c ]色烯-6(7 H )-酮。值得注意的是,该方案通过在单锅中激活非反应性酯部分,能够在无金属反应条件下构建两个 C-C 键和一个 C-O 键。中间体茚醇酯的分离可能表明通过烯酸酯的邻近双键与邻羰基部分进行自分子内环加成,随后用苯酚进行亲电攻击以及随后的环缩合途径。此外,还检查了光物理性质。
更新日期:2023-09-18
中文翻译:

酸介导的茚并[2,1-c]色烯-6(7H)-酮的级联环化途径
开发温和有效的合成策略,从容易获得的起始材料开始生产重要的分子,在有机合成中是必不可少的。在此,我们提出了一种三氟甲磺酸驱动的双环化途径,从简单的2-甲酰基(或2-酰基)肉桂酸酯和酚类生产功能化的茚并[2,1- c ]色烯-6(7 H )-酮。值得注意的是,该方案通过在单锅中激活非反应性酯部分,能够在无金属反应条件下构建两个 C-C 键和一个 C-O 键。中间体茚醇酯的分离可能表明通过烯酸酯的邻近双键与邻羰基部分进行自分子内环加成,随后用苯酚进行亲电攻击以及随后的环缩合途径。此外,还检查了光物理性质。









































 京公网安备 11010802027423号
京公网安备 11010802027423号