Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2023-09-16 , DOI: 10.1016/j.jhazmat.2023.132560 Jiawen Zhou 1 , Rebekah E T Moore 2 , Mark Rehkämper 2 , Katharina Kreissig 2 , Barry Coles 2 , Longhua Wu 1 , Yongming Luo 1 , Peter Christie 1
|
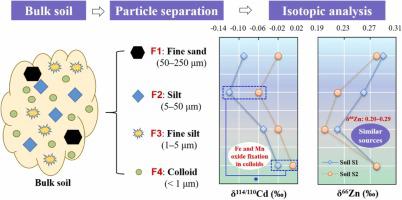
Soil particle size may significantly affect metal distribution and stable isotopic behavior. Here, two soils were separated into four particle size fractions, namely fine sand, silt, fine silt, and colloidal particles and used to determine cadmium (Cd) and zinc (Zn) concentrations and isotope compositions. Concentrations of Cd and Zn were generally enriched in the finer particles and positively correlated with the iron (Fe) and manganese (Mn) oxide contents. However, Cd concentration in the fine sand was higher than in the silt fraction due to the higher soil organic matter contents in the former particle fraction. The maximum δ114/110Cd value was found in the colloidal particles (−0.02 and 0.01‰) of both soils while the minimum was in the silt particles (−0.12 and  0.06‰). Incorporation into the mineral lattice of Fe and Mn oxides is suggested to explain the slight enrichment of heavy Cd isotopes in the colloidal fraction. The similar δ66Zn values of the four particle fractions (0.20–0.29‰ with a mean of 0.25‰) indicate similar Zn sources in different particle sizes. Metal isotopic fingerprint of different soil particle size fractions provides further insight into underlying metal retention mechanisms within soil micro-zones and helps in tracing metal sources and biogeochemical processes.
0.06‰). Incorporation into the mineral lattice of Fe and Mn oxides is suggested to explain the slight enrichment of heavy Cd isotopes in the colloidal fraction. The similar δ66Zn values of the four particle fractions (0.20–0.29‰ with a mean of 0.25‰) indicate similar Zn sources in different particle sizes. Metal isotopic fingerprint of different soil particle size fractions provides further insight into underlying metal retention mechanisms within soil micro-zones and helps in tracing metal sources and biogeochemical processes.
Environmental implication
Cadmium (Cd) was a highly toxic metal which ranks the first in the percentage of Chinese farmland soil samples (7.0%) exceeding the soil environmental quality standard and zinc (Zn) often co-contaminated with Cd in the vicinity of mining and smelting area. Here isotopic technique combined with metal speciation analysis was used to reveal Cd and Zn sources and retention mechanisms in soil particles with different sizes. Our findings suggest the advantages of metal stable isotopic tool in studying the processes controlling metal migration in natural environments and the processes underlying the formation, aggregation and migration of metal-containing nanoparticles.




















































 京公网安备 11010802027423号
京公网安备 11010802027423号