Journal of Drug Delivery Science and Technology ( IF 4.5 ) Pub Date : 2023-09-16 , DOI: 10.1016/j.jddst.2023.104955 Bianca Costa Bernardo Port , Débora Fretes Argenta , Douglas Santos Porto , Gabriela Schneider Rauber , Isabella Dai Prá Zuchi , Izabella Thaís Silva , Thiago Caon
|
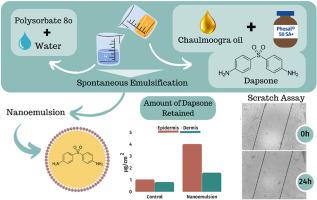
Drugs used to treat leprosy such as dapsone (DAP) can result in various adverse effects when administered orally. The disease-causing pathogen has a preferential distribution in peripheral regions of the body and symptoms are manifested primarily in the skin and peripheral nerves. This aspect motivated, in this study, the development of topical formulations containing DAP. Nanoemulsions (NEs) were selected as drug carriers aiming to enhance cutaneous permeation and sustain the drug release. Chaulmoogra oil (CH) was added to the oily nucleus of the NEs considering its history of use in leprosy and its potential healing effect in the skin lesions caused by Mycobacterium leprae. CH effect on formulation characteristics (size, PDI, surface load and stability) and epithelial cell migration was evaluated. CH-based NEs showed to be more monodisperse than NEs without CH (PDI ≤0.26 vs. 0.37). The droplet diameter increased from 189.7 to 227.0 nm when the CH content ranged from 2 to 4%, respectively. Hot-stage analyses and accelerated stability studies revealed that the CH improves both the physical and thermal stability of these nanocarriers. NEs with higher CH content presented greater interaction with the human skin in spectroscopic analyses, which was confirmed by permeation studies. These same formulations increased the amount of DAP retained in the skin (3.40 μg cm−2 in the epidermis and 1.60 μg cm−2 in the dermis) when compared to the nanoemulsion (NE) containing the lowest CH content (2.01 μg cm−2 in the epidermis and 1.06 μg cm−2 in the dermis). Another positive effect is that free and nanoencapsulated CH improved cell migration, which would be useful to treat skin lesions that commonly appear in patients with leprosy. Both free CH and NE provided a wound closure of 70 and 69%, respectively. In summary, the presence of CH is able to improve the anti-leprosy formulation characteristics and provide different benefits to patients.
中文翻译:

用于麻风病治疗的 Chaulmoogra 油基纳米乳剂:氨苯砜的案例研究
用于治疗麻风病的药物(例如氨苯砜(DAP))口服给药时可能会导致各种不良反应。致病病原体优先分布在身体的周围区域,症状主要表现在皮肤和周围神经。在本研究中,这一方面推动了含有 DAP 的局部制剂的开发。选择纳米乳剂(NE)作为药物载体,旨在增强皮肤渗透并维持药物释放。考虑到 Chaulmoogra 油 (CH) 在麻风病中的使用历史及其对麻风分枝杆菌引起的皮肤损伤的潜在治愈作用,将 Chaulmoogra 油 (CH) 添加到 NE 的油性核中。评估了 CH 对制剂特性(尺寸、PDI、表面负载和稳定性)和上皮细胞迁移的影响。基于 CH 的 NE 比不含 CH 的 NE 更具有单分散性(PDI ≤0.26 vs. 0.37)。当CH含量在2%~4%范围内时,液滴直径分别从189.7 nm增加到227.0 nm 。热阶段分析和加速稳定性研究表明,CH 提高了这些纳米载体的物理和热稳定性。CH 含量较高的 NE 在光谱分析中与人体皮肤的相互作用更强,这一点已被渗透研究所证实。这些相同的配方增加了皮肤中保留的 DAP 量(表皮中为3.40 μg cm -2 ,表皮中为 1.60 μg cm -2与含有最低 CH 含量的纳米乳液 (NE)(表皮中2.01 μg cm -2 ,真皮中1.06 μg cm -2 )相比。另一个积极作用是游离和纳米封装的 CH 改善了细胞迁移,这将有助于治疗麻风患者常见的皮肤病变。游离 CH 和 NE 分别提供 70% 和 69% 的伤口闭合。总之,CH的存在能够改善抗麻风制剂的特性并为患者提供不同的益处。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号