Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Anti-Breast Cancer Potency of Mono- and Bis-(pyrazolyl[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine) Derivatives as EGFR/CDK-2 Target Inhibitors
ACS Omega ( IF 3.7 ) Pub Date : 2023-09-11 , DOI: 10.1021/acsomega.3c05309 Mostafa E Salem 1, 2 , Esraa M Mahrous 1 , Eman A Ragab 1 , Mohamed S Nafie 3, 4 , Kamal M Dawood 1
ACS Omega ( IF 3.7 ) Pub Date : 2023-09-11 , DOI: 10.1021/acsomega.3c05309 Mostafa E Salem 1, 2 , Esraa M Mahrous 1 , Eman A Ragab 1 , Mohamed S Nafie 3, 4 , Kamal M Dawood 1
Affiliation
|
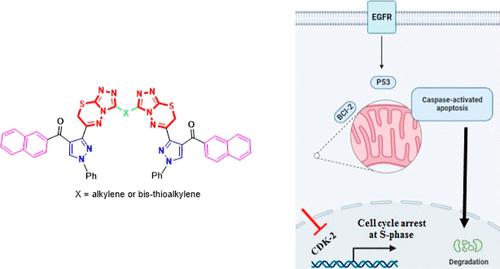
The target mono- and bis-(6-pyrazolyltriazolo-thiadiazine) derivatives 4a-c and 6a-d were synthesized using a straightforward protocol via reaction of 3-bromoacetylpyrazole 2 with 4-amino-s-triazole-3-thiols 3a-c and bis(4-amino-5-mercapto-s-triazol-3-yl)alkanes 5a-d, respectively. The bis(6-pyrazolyl-s-triazolo[3,4-b][1,3,4]thiadiazine) derivatives 8a,b and 10 were also constructed by reaction of the triazolo[3,4-b][1,3,4]thiadiazine-3-thiol 4c with the proper dibromo compounds 7a,b and 9, respectively. Structures of the new substances were determined by spectroscopic and analytical data. Compounds 4b, 4c, and 6a showed potent cytotoxicity against MCF-7 (IC50 = 3.16, 2.74, and 0.39 μM, respectively) and were safe against the MCF-10A cells. Compounds 4b, 4c, and 6a also showed promising dual EGFR and CDK-2 inhibition activities, particularly 6a was the most effective (IC50 = 19.6 and 87.9 nM, respectively), better than Erlotinib and Roscovitine. Compound 6a treatment induced EGFR and CDK-2 enzyme inhibition by 97.18% and 94.11%, respectively, at 10 μM (the highest concentration). Compound 6a notably induced cell apoptosis in MCF-7 cells, increasing the cell population by total apoptosis 43.3% compared to 1.29% for the untreated control group, increasing the cell population at the S-phase by 39.2% compared to 18.6% (control).
中文翻译:

作为 EGFR/CDK-2 靶抑制剂的单和双(吡唑基[1,2,4]三唑并[3,4-b][1,3,4]噻二嗪)衍生物的合成及其抗乳腺癌功效
使用简单的方案,通过 3-溴乙酰基吡唑2与 4-氨基-s-三唑-3-硫醇3a-c反应,合成目标单-和双-(6-吡唑基三唑基-噻二嗪)衍生物4a-c和6a-d和双(4-氨基-5-巯基-s-三唑-3-基)烷烃5a-d。双(6-吡唑基-s-三唑并[3,4- b ][1,3,4]噻二嗪)衍生物8a、b和10也是通过三唑并[3,4- b ][1, 3,4]噻二嗪-3-硫醇4c分别与适当的二溴化合物7a、b和9。新物质的结构通过光谱和分析数据确定。化合物4b、4c和6a对MCF-7表现出有效的细胞毒性(IC 50分别为3.16、2.74和0.39 μM),并且对MCF-10A细胞是安全的。化合物4b、4c和6a也显示出有希望的EGFR和CDK-2双重抑制活性,特别是6a是最有效的(IC 50分别为19.6和87.9 nM),优于Erlotinib和Roscovitine。10 μM(最高浓度)的化合物6a处理分别诱导 EGFR 和 CDK-2 酶抑制 97.18% 和 94.11%。化合物6a显着诱导 MCF-7 细胞中的细胞凋亡,与未处理对照组的 1.29% 相比,总凋亡细胞群增加了 43.3%,与 18.6%(对照)相比,S 期细胞群增加了 39.2% 。
更新日期:2023-09-11
中文翻译:

作为 EGFR/CDK-2 靶抑制剂的单和双(吡唑基[1,2,4]三唑并[3,4-b][1,3,4]噻二嗪)衍生物的合成及其抗乳腺癌功效
使用简单的方案,通过 3-溴乙酰基吡唑2与 4-氨基-s-三唑-3-硫醇3a-c反应,合成目标单-和双-(6-吡唑基三唑基-噻二嗪)衍生物4a-c和6a-d和双(4-氨基-5-巯基-s-三唑-3-基)烷烃5a-d。双(6-吡唑基-s-三唑并[3,4- b ][1,3,4]噻二嗪)衍生物8a、b和10也是通过三唑并[3,4- b ][1, 3,4]噻二嗪-3-硫醇4c分别与适当的二溴化合物7a、b和9。新物质的结构通过光谱和分析数据确定。化合物4b、4c和6a对MCF-7表现出有效的细胞毒性(IC 50分别为3.16、2.74和0.39 μM),并且对MCF-10A细胞是安全的。化合物4b、4c和6a也显示出有希望的EGFR和CDK-2双重抑制活性,特别是6a是最有效的(IC 50分别为19.6和87.9 nM),优于Erlotinib和Roscovitine。10 μM(最高浓度)的化合物6a处理分别诱导 EGFR 和 CDK-2 酶抑制 97.18% 和 94.11%。化合物6a显着诱导 MCF-7 细胞中的细胞凋亡,与未处理对照组的 1.29% 相比,总凋亡细胞群增加了 43.3%,与 18.6%(对照)相比,S 期细胞群增加了 39.2% 。






























 京公网安备 11010802027423号
京公网安备 11010802027423号