当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioconvergent 6π Electrocyclization Enabled by Photoredox Racemization
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-09-12 , DOI: 10.1021/jacs.3c06227 Sebastijan Ričko 1, 2 , René Slot Bitsch 1 , Mikk Kaasik 1 , Jan Otevřel 1 , Mikkel Højgaard Madsen 1 , Anna Keimer 1 , Karl Anker Jørgensen 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-09-12 , DOI: 10.1021/jacs.3c06227 Sebastijan Ričko 1, 2 , René Slot Bitsch 1 , Mikk Kaasik 1 , Jan Otevřel 1 , Mikkel Højgaard Madsen 1 , Anna Keimer 1 , Karl Anker Jørgensen 1
Affiliation
|
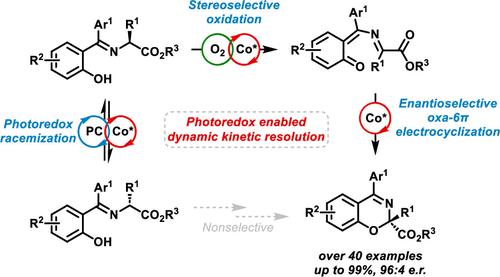
This study presents a novel photoredox-enabled enantioconvergent catalytic strategy used to construct chiral 2H-1,3-benzoxazines via an unprecedented oxa-6π electrocyclization utilizing racemic α-substituted glycinates as substrates. The approach leverages a cobalt-based chiral Lewis acid catalyst, which promotes the transformation under thermal or photoredox conditions. While the thermal reaction selectively converts only the (S)-configured glycinates into enantioenriched 2H-1,3-benzoxazines (up to 96:4 e.r.), the addition of 0.5 mol % of a commercially available iridium photocatalyst under visible light irradiation transforms the reaction into an enantioconvergent process. Detailed mechanistic and time course studies of optically pure α-deuterated substrates revealed the presence of an enantiospecific kinetic isotope effect, which helped to clarify the role of both the photo- and chiral Lewis acid catalyst in the reaction sequence. In this dual catalytic system, the photocatalyst promotes a dynamic interconversion between the substrate enantiomers─a process not accessible via ground-state chemistry─while the chiral Lewis acid selectively transforms only the (S)-configured substrates. Further mechanistic evidence for the proposed mechanism is provided by linear free energy relationship analysis, which suggests that the stereodetermining step involves a 6π electrocyclization under both thermal and photoredox conditions.
中文翻译:

光氧化还原外消旋化实现对映收敛 6π 电环化
这项研究提出了一种新型的光氧化还原对映催化策略,用于利用外消旋 α-取代甘氨酸盐作为底物,通过前所未有的 oxa-6π 电环化来构建手性 2 H -1,3-苯并恶嗪。该方法利用钴基手性路易斯酸催化剂,促进热或光氧化还原条件下的转化。虽然热反应选择性地将 ( S ) 配置的甘氨酸盐转化为对映体富集的 2 H -1,3-苯并恶嗪(高达 96:4 er),但在可见光照射下添加 0.5 mol% 的市售铱光催化剂会发生转化。反应转变为对映体收敛过程。对光学纯α-氘化底物的详细机理和时间过程研究揭示了对映特异性动力学同位素效应的存在,这有助于阐明光催化剂和手性路易斯酸催化剂在反应序列中的作用。在这个双催化系统中,光催化剂促进底物对映体之间的动态相互转化——这是基态化学无法实现的过程——而手性路易斯酸仅选择性地转化(S)配置的底物。线性自由能关系分析为所提出的机制提供了进一步的机械证据,这表明立体决定步骤涉及热和光氧化还原条件下的 6π 电环化。
更新日期:2023-09-12
中文翻译:

光氧化还原外消旋化实现对映收敛 6π 电环化
这项研究提出了一种新型的光氧化还原对映催化策略,用于利用外消旋 α-取代甘氨酸盐作为底物,通过前所未有的 oxa-6π 电环化来构建手性 2 H -1,3-苯并恶嗪。该方法利用钴基手性路易斯酸催化剂,促进热或光氧化还原条件下的转化。虽然热反应选择性地将 ( S ) 配置的甘氨酸盐转化为对映体富集的 2 H -1,3-苯并恶嗪(高达 96:4 er),但在可见光照射下添加 0.5 mol% 的市售铱光催化剂会发生转化。反应转变为对映体收敛过程。对光学纯α-氘化底物的详细机理和时间过程研究揭示了对映特异性动力学同位素效应的存在,这有助于阐明光催化剂和手性路易斯酸催化剂在反应序列中的作用。在这个双催化系统中,光催化剂促进底物对映体之间的动态相互转化——这是基态化学无法实现的过程——而手性路易斯酸仅选择性地转化(S)配置的底物。线性自由能关系分析为所提出的机制提供了进一步的机械证据,这表明立体决定步骤涉及热和光氧化还原条件下的 6π 电环化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号