当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ROS-Responsive Self-Degradable DNA Nanogels for Targeted Anticancer Drug Delivery
ACS Macro Letters ( IF 5.1 ) Pub Date : 2023-09-15 , DOI: 10.1021/acsmacrolett.3c00442 Xiaonong Zhang 1 , Peng Zhang 1, 2 , Chunsheng Xiao 1 , Xuesi Chen 1
ACS Macro Letters ( IF 5.1 ) Pub Date : 2023-09-15 , DOI: 10.1021/acsmacrolett.3c00442 Xiaonong Zhang 1 , Peng Zhang 1, 2 , Chunsheng Xiao 1 , Xuesi Chen 1
Affiliation
|
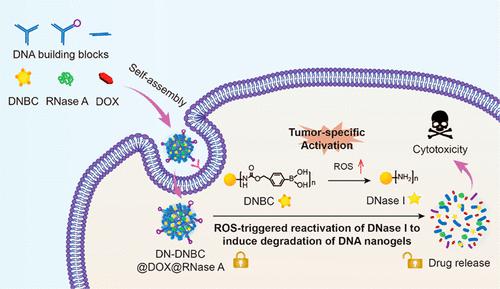
Here, a reactive oxygen species (ROS)-responsive targeted anticancer drug delivery system was developed by embedding a nitrophenyl tetramethyl-dioxaborolanyl benzyl carbamate (NBC)-modified deoxyribonuclease I (DNase I) in a DNase-degradable aptamer-based DNA nanogel. The DNA nanogel was formed by hybridization of three types of building blocks, namely, Y-shaped monomer 1 with three sticky ends, Y-shaped monomer 2 with two sticky ends and an aptamer end, and a DNA linker with two sticky ends. Single doxorubicin (DOX) or ribonuclease A (RNase A) as well as the combination of DOX and RNase A were effectively loaded into the nanogels, wherein DOX was embedded into DNA skeleton, while RNase A was encapsulated into nanogel matrix. The blocked enzymatic activity of DNase I due to NBC modification could be restored upon intracellular ROS-triggered NBC deprotection, resulting in self-degradation of the nanogels to release both DOX and RNase A. Consequently, the DOX and RNase A coloaded nanogels significantly inhibited the proliferation of MCF-7 cells through a synergistic effect. To sum up, this DNA-based drug delivery system with ROS-responsive self-degradation properties should be promising for application in targeted and synergistic cancer therapy.
中文翻译:

用于靶向抗癌药物递送的 ROS 响应性自降解 DNA 纳米凝胶
在这里,通过将硝基苯基四甲基二氧硼杂硼基苄基氨基甲酸酯(NBC)修饰的脱氧核糖核酸酶 I(DNase I)嵌入基于 DNase 可降解适体的 DNA 纳米凝胶中,开发了一种活性氧(ROS)响应的靶向抗癌药物递送系统。DNA纳米凝胶由三种类型的构件杂交形成,即具有三个粘性末端的Y形单体1、具有两个粘性末端和适体末端的Y形单体2以及具有两个粘性末端的DNA接头。单一阿霉素(DOX)或核糖核酸酶A(RNase A)以及DOX和RNase A的组合被有效负载到纳米凝胶中,其中DOX嵌入DNA骨架,而RNase A封装在纳米凝胶基质中。由于 NBC 修饰而被阻断的 DNase I 酶活性可以在细胞内 ROS 触发的 NBC 去保护后恢复,导致纳米凝胶自我降解,释放 DOX 和 RNase A。因此,DOX 和 RNase A 共负载纳米凝胶显着抑制通过协同作用促进MCF-7细胞的增殖。综上所述,这种具有ROS响应自降解特性的基于DNA的药物递送系统应该有望在靶向和协同癌症治疗中得到应用。
更新日期:2023-09-15
中文翻译:

用于靶向抗癌药物递送的 ROS 响应性自降解 DNA 纳米凝胶
在这里,通过将硝基苯基四甲基二氧硼杂硼基苄基氨基甲酸酯(NBC)修饰的脱氧核糖核酸酶 I(DNase I)嵌入基于 DNase 可降解适体的 DNA 纳米凝胶中,开发了一种活性氧(ROS)响应的靶向抗癌药物递送系统。DNA纳米凝胶由三种类型的构件杂交形成,即具有三个粘性末端的Y形单体1、具有两个粘性末端和适体末端的Y形单体2以及具有两个粘性末端的DNA接头。单一阿霉素(DOX)或核糖核酸酶A(RNase A)以及DOX和RNase A的组合被有效负载到纳米凝胶中,其中DOX嵌入DNA骨架,而RNase A封装在纳米凝胶基质中。由于 NBC 修饰而被阻断的 DNase I 酶活性可以在细胞内 ROS 触发的 NBC 去保护后恢复,导致纳米凝胶自我降解,释放 DOX 和 RNase A。因此,DOX 和 RNase A 共负载纳米凝胶显着抑制通过协同作用促进MCF-7细胞的增殖。综上所述,这种具有ROS响应自降解特性的基于DNA的药物递送系统应该有望在靶向和协同癌症治疗中得到应用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号