当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electron-Transfer-Enabled Concerted Nucleophilic Fluorination of Azaarenes: Selective C–H Fluorination of Quinolines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-09-11 , DOI: 10.1021/jacs.3c07119 Li Zhang 1 , Jiyao Yan 1, 2 , Dilgam Ahmadli 1, 2 , Zikuan Wang 1 , Tobias Ritter 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-09-11 , DOI: 10.1021/jacs.3c07119 Li Zhang 1 , Jiyao Yan 1, 2 , Dilgam Ahmadli 1, 2 , Zikuan Wang 1 , Tobias Ritter 1
Affiliation
|
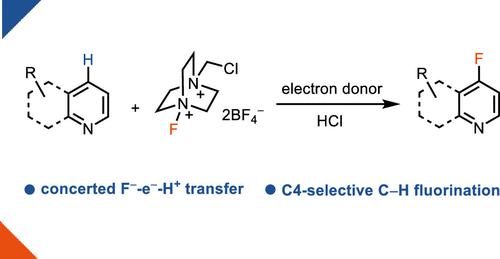
Direct C–H fluorination is an efficient strategy to construct aromatic C–F bonds, but the cleavage of specific C–H bonds in the presence of other functional groups and the high barrier of C–F bond formation make the transformation challenging. Progress for the electrophilic fluorination of arenes has been reported, but a similar transformation for electron-deficient azaarenes has remained elusive due to the high energy of the corresponding Wheland intermediates. Nucleophilic fluorination of electron-deficient azaarenes is difficult owing to the identity of the Meisenheimer intermediate after fluoride attack, from which fluoride elimination to regenerate the substrate is favored over hydride elimination to form the product. Herein, we report a new concept for C–H nucleophilic fluorination without the formation of azaarene Meisenheimer intermediates through a chain process with an asynchronous concerted F–-e–-H+ transfer. The concerted nucleophilic aromatic substitution strategy allows for the first successful nucleophilic oxidative fluorination of quinolines.
中文翻译:

氮杂芳烃的电子转移协同亲核氟化:喹啉的选择性 C-H 氟化
直接C-H氟化是构建芳香族C-F键的有效策略,但在其他官能团存在的情况下特定C-H键的断裂以及C-F键形成的高势垒使得转化具有挑战性。芳烃亲电氟化的进展已有报道,但由于相应的 Wheland 中间体的高能量,缺电子氮杂芳烃的类似转化仍然难以实现。由于氟化物攻击后迈森海默中间体的特性,缺电子氮杂芳烃的亲核氟化是困难的,其中氟化物消除以再生底物比氢化物消除形成产物更有利。在此,我们报告了一种新概念,即通过异步协同 F – -e – -H +转移的链式过程,不形成氮杂芳烃 Meisenheimer 中间体的 C–H 亲核氟化。协调一致的亲核芳族取代策略使得喹啉首次成功发生亲核氧化氟化。
更新日期:2023-09-11
中文翻译:

氮杂芳烃的电子转移协同亲核氟化:喹啉的选择性 C-H 氟化
直接C-H氟化是构建芳香族C-F键的有效策略,但在其他官能团存在的情况下特定C-H键的断裂以及C-F键形成的高势垒使得转化具有挑战性。芳烃亲电氟化的进展已有报道,但由于相应的 Wheland 中间体的高能量,缺电子氮杂芳烃的类似转化仍然难以实现。由于氟化物攻击后迈森海默中间体的特性,缺电子氮杂芳烃的亲核氟化是困难的,其中氟化物消除以再生底物比氢化物消除形成产物更有利。在此,我们报告了一种新概念,即通过异步协同 F – -e – -H +转移的链式过程,不形成氮杂芳烃 Meisenheimer 中间体的 C–H 亲核氟化。协调一致的亲核芳族取代策略使得喹啉首次成功发生亲核氧化氟化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号