当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ionization, intrinsic basicity, and intrinsic acidity of unsaturated diols of astrochemical interest: 1,1- and 1,2-ethenediol: A theoretical survey
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2023-09-12 , DOI: 10.1002/jcc.27223 Otilia Mó 1 , Al Mokhtar Lamsabhi 1 , Jean-Claude Guillemin 2 , Manuel Yáñez 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2023-09-12 , DOI: 10.1002/jcc.27223 Otilia Mó 1 , Al Mokhtar Lamsabhi 1 , Jean-Claude Guillemin 2 , Manuel Yáñez 1
Affiliation
|
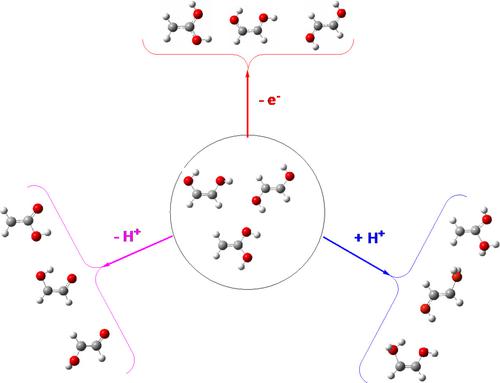
The structure, stability, and bonding characteristics of 1,1- and 1,2-ethenediol, their radical cations, and their protonated and deprotonated species were investigated using high-level ab initio G4 calculations. The electron density of all the neutral and charged systems investigated was analyzed using the QTAIM, ELF, and NBO approaches. The vertical ionization potential (IP) of the five stable tautomers of 1,2-ethenediol and the two stable tautomers of 1,1-ethenediol go from 11.81 to 12.27 eV, whereas the adiabatic ones go from 11.00 to 11.72 eV. The adiabatic ionization leads to a significant charge delocalization along the O-C-C-O skeleton. The most stable protonated form of (Z)-1,2-ethenediol can be reached by the protonation of both the anti-anti and the syn-anti conformers, whereas the most stable deprotonated form arises only from the syn-anti one. Both charged species are extra-stabilized by the formation of an O-H···O intramolecular hydrogen bond (IHB) which is not found in the neutral system. (Z)-1,2-ethenediol is predicted to be less stable, less basic, and more acidic than its cis-glycolaldehyde isomer. The most stable protonated species of (E)-1,2-ethenediol comes from its syn-syn conformer, although the anti-anti conformer is the most basic one. Contrarily, the three conformers yield a common deprotonated species, so their acidity follows exactly their relative stability. Again, the (E)-1,2-ethenediol is predicted to be less stable, less basic, and more acidic than its trans-glycolaldehyde isomer. Neither the neutral nor the protonated or the deprotonated forms of 1,1-ethenediol show the formation of any O-H···O IHB. The most stable protonated species is formed by the protonation of any of the two tautomers, but the most stable deprotonated form arises exclusively from the syn-anti neutral conformer. The conformers of 1,1-ethenediol are much less stable and significantly less basic than their isomer, acetic acid, and only slightly more acidic.
中文翻译:

天文化学感兴趣的不饱和二醇的电离、固有碱性和固有酸性:1,1-和1,2-乙烯二醇:理论调查
使用高级从头算 G4 计算研究了 1,1- 和 1,2- 乙烯二醇、其自由基阳离子及其质子化和去质子化物质的结构、稳定性和键合特性。使用 QTAIM、ELF 和 NBO 方法分析了所研究的所有中性和带电系统的电子密度。 1,2-乙烯二醇的五个稳定互变异构体和1,1-乙烯二醇的两个稳定互变异构体的垂直电离势(IP)从11.81到12.27 eV,而绝热互变异构体从11.00到11.72 eV。绝热电离导致沿 OCCO 骨架的显着电荷离域。 ( Z )-1,2-乙烯二醇最稳定的质子化形式可以通过抗-抗和顺-反构象异构体的质子化来实现,而最稳定的去质子化形式仅由顺-反构象异构体产生。两种带电物质通过形成 OH·O 分子内氢键 (IHB) 而获得额外稳定,而中性体系中没有这种氢键。 ( Z )-1,2-乙烯二醇预计比其顺式乙醇醛异构体更不稳定、碱性更弱且酸性更强。 ( E )-1,2-乙烯二醇最稳定的质子化种类来自其顺-顺构象异构体,尽管反反构象异构体是最基本的构象异构体。相反,这三种构象异构体产生共同的去质子化物质,因此它们的酸性完全遵循它们的相对稳定性。同样,预计 ( E )-1,2-乙烯二醇比其反式乙醇醛异构体稳定性较差、碱性较差且酸性更强。 1,1-乙烯二醇的中性形式、质子化形式或去质子化形式均未显示出任何OH·O IHB 的形成。最稳定的质子化形式是由两个互变异构体中任何一个的质子化形成的,但最稳定的去质子化形式完全由顺-反中性构象异构体产生。 1,1-乙烯二醇的构象异构体比其异构体乙酸稳定性差得多,碱性显着降低,并且酸性仅稍高一些。
更新日期:2023-09-12
中文翻译:

天文化学感兴趣的不饱和二醇的电离、固有碱性和固有酸性:1,1-和1,2-乙烯二醇:理论调查
使用高级从头算 G4 计算研究了 1,1- 和 1,2- 乙烯二醇、其自由基阳离子及其质子化和去质子化物质的结构、稳定性和键合特性。使用 QTAIM、ELF 和 NBO 方法分析了所研究的所有中性和带电系统的电子密度。 1,2-乙烯二醇的五个稳定互变异构体和1,1-乙烯二醇的两个稳定互变异构体的垂直电离势(IP)从11.81到12.27 eV,而绝热互变异构体从11.00到11.72 eV。绝热电离导致沿 OCCO 骨架的显着电荷离域。 ( Z )-1,2-乙烯二醇最稳定的质子化形式可以通过抗-抗和顺-反构象异构体的质子化来实现,而最稳定的去质子化形式仅由顺-反构象异构体产生。两种带电物质通过形成 OH·O 分子内氢键 (IHB) 而获得额外稳定,而中性体系中没有这种氢键。 ( Z )-1,2-乙烯二醇预计比其顺式乙醇醛异构体更不稳定、碱性更弱且酸性更强。 ( E )-1,2-乙烯二醇最稳定的质子化种类来自其顺-顺构象异构体,尽管反反构象异构体是最基本的构象异构体。相反,这三种构象异构体产生共同的去质子化物质,因此它们的酸性完全遵循它们的相对稳定性。同样,预计 ( E )-1,2-乙烯二醇比其反式乙醇醛异构体稳定性较差、碱性较差且酸性更强。 1,1-乙烯二醇的中性形式、质子化形式或去质子化形式均未显示出任何OH·O IHB 的形成。最稳定的质子化形式是由两个互变异构体中任何一个的质子化形成的,但最稳定的去质子化形式完全由顺-反中性构象异构体产生。 1,1-乙烯二醇的构象异构体比其异构体乙酸稳定性差得多,碱性显着降低,并且酸性仅稍高一些。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号