European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-09-12 , DOI: 10.1016/j.ejmech.2023.115803 Filippo Basagni 1 , Jose A Ortega 2 , Sine M Bertozzi 3 , Andrea Armirotti 3 , Maria Summa 4 , Rosalia Bertorelli 4 , Manuela Bartolini 1 , Ian R Mellor 5 , Martina Bedeschi 6 , Giovanni Bottegoni 7 , Vittorio Lembo 8 , Anna Minarini 1 , Andrea Cavalli 9 , Michela Rosini 1
|
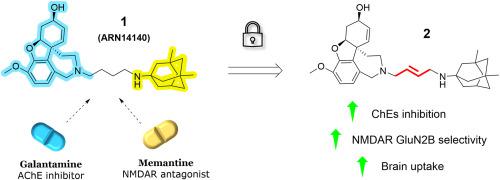
Neurodegenerative processes characterizing Alzheimer's disease (AD) are strictly related to the impairment of cholinergic and glutamatergic neurotransmitter systems which provoke synaptic loss. These experimental evidences still represent the foundation of the actual standard-of-care treatment for AD, albeit palliative, consisting on the coadministration of an acetylcholinesterase inhibitor and the NMDAR antagonist memantine. In looking for more effective treatments, we previously developed a series of galantamine-memantine hybrids where compound 1 (ARN14140) emerged with the best-balanced action toward the targets of interest paired to neuroprotective efficacy in a murine AD model. Unfortunately, it showed a suboptimal pharmacokinetic profile, which required intracerebroventricular administration for in vivo studies. In this work we designed and synthesized new hybrids with fewer rotatable bonds, which is related to higher brain exposure. Particularly, compound 2, bearing a double bond in the tether, ameliorated the biological profile of compound 1 in in vitro studies, increasing cholinesterases inhibitory potencies and selective antagonism toward excitotoxic-related GluN1/2B NMDAR over beneficial GluN1/2A NMDAR. Furthermore, it showed increased plasma stability and comparable microsomal stability in vitro, paired with lower half-life and faster clearance in vivo. Remarkably, pharmacokinetic evaluations of compound 2 showed a promising increase in brain uptake in comparison to compound 1, representing the starting point for further chemical optimizations.
中文翻译:

加兰他敏-美金刚杂合药物治疗阿尔茨海默病:接头刚性对生物活性和药代动力学特性的影响
阿尔茨海默病(AD)的神经退行性过程与胆碱能和谷氨酸能神经递质系统的损伤密切相关,后者会引起突触损失。这些实验证据仍然代表了 AD 实际标准护理治疗的基础,尽管是姑息性的,包括乙酰胆碱酯酶抑制剂和 NMDAR 拮抗剂美金刚的共同给药。为了寻找更有效的治疗方法,我们之前开发了一系列加兰他敏-美金刚杂合药物,其中化合物1 (ARN14140) 在小鼠 AD 模型中对目标靶标具有最佳平衡的作用,并具有神经保护功效。不幸的是,它表现出次优的药代动力学特征,需要脑室内给药进行体内研究。在这项工作中,我们设计并合成了具有较少可旋转键的新杂交体,这与较高的大脑暴露有关。特别地,在体外研究中,化合物2在系链中带有双键,改善了化合物1的生物学特征,增加了胆碱酯酶抑制效力,并且相对于有益的GluN1/2A NMDAR对兴奋性毒性相关的GluN1/2B NMDAR具有选择性拮抗作用。此外,它在体外表现出更高的血浆稳定性和相当的微粒体稳定性,同时具有更低的半衰期和更快的体内清除率。值得注意的是,与化合物1相比,化合物2的药代动力学评估显示脑摄取量有望增加,这代表了进一步化学优化的起点。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号