当前位置:
X-MOL 学术
›
Small Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Manipulating the Solvation Structure and Interface via a Bio-Based Green Additive for Highly Stable Zn Metal Anode
Small Methods ( IF 10.7 ) Pub Date : 2023-09-10 , DOI: 10.1002/smtd.202300804
Yan Wang 1 , Xiaohui Zeng 2 , Haiji Huang 1 , Dongmei Xie 1 , Jianyang Sun 1 , Jiachang Zhao 1 , Yichuan Rui 1 , Jinguo Wang 1 , Jodie A Yuwono 3 , Jianfeng Mao 3
Small Methods ( IF 10.7 ) Pub Date : 2023-09-10 , DOI: 10.1002/smtd.202300804
Yan Wang 1 , Xiaohui Zeng 2 , Haiji Huang 1 , Dongmei Xie 1 , Jianyang Sun 1 , Jiachang Zhao 1 , Yichuan Rui 1 , Jinguo Wang 1 , Jodie A Yuwono 3 , Jianfeng Mao 3
Affiliation
|
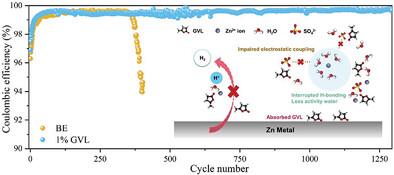
The practical application of aqueous zinc-ion batteries (AZIBs) is limited by serious side reactions, such as the hydrogen evolution reaction and Zn dendrite growth. Here, the study proposes a novel adoption of a biodegradable electrolyte additive, γ-Valerolactone (GVL), with only 1 vol.% addition (GVL-to-H2O volume ratio) to enable a stable Zn metal anode. The combination of experimental characterizations and theoretical calculations verifies that the green GVL additive can competitively engage the solvated structure of Zn2+ via replacing a H2O molecule from [Zn(H2O)6]2+, which can efficiently reduce the reactivity of water and inhibit the subsequent side reactions. Additionally, GVL molecules are preferentially adsorbed on the surface of Zn to regulate the uniform Zn deposition and suppress the Zn dendrite growth. Consequently, the Zn anode exhibits boosted stability with ultralong cycle lifespan (over 3500 h) and high reversibility with 99.69% Coulombic efficiency. The Zn||MnO2 full batteries with ZnSO4-GVL electrolyte show a high capacity of 219 mAh g−1 at 0.5 A g−1 and improved capacity retention of 78% after 550 cycles. This work provides inspiration on bio-based electrolyte additives for aqueous battery chemistry and promotes the practical application of AZIBs.
中文翻译:

通过生物基绿色添加剂控制溶剂化结构和界面以获得高度稳定的锌金属阳极
水系锌离子电池(AZIB)的实际应用受到严重副反应的限制,例如析氢反应和锌枝晶生长。在此,该研究提出了一种新的可生物降解电解质添加剂γ-戊内酯(GVL)的采用,仅添加1体积%(GVL与H 2 O的体积比)即可实现稳定的锌金属阳极。实验表征和理论计算相结合,验证了绿色GVL添加剂可以通过替换[Zn(H 2 O) 6 ] 2+中的H 2 O分子,竞争性地参与Zn 2+的溶剂化结构,从而有效降低反应活性水并抑制随后的副反应。此外,GVL分子优先吸附在Zn表面,以调节Zn的均匀沉积并抑制Zn枝晶的生长。因此,锌阳极表现出更高的稳定性、超长的循环寿命(超过 3500 小时)和高可逆性,库仑效率高达 99.69%。采用ZnSO 4 -GVL电解质的Zn||MnO 2全电池在0.5 A g -1下表现出219 mAh g -1的高容量,并且在550次循环后容量保持率提高到78%。这项工作为水系电池化学的生物基电解质添加剂提供了灵感,并促进了AZIBs的实际应用。
更新日期:2023-09-10
中文翻译:

通过生物基绿色添加剂控制溶剂化结构和界面以获得高度稳定的锌金属阳极
水系锌离子电池(AZIB)的实际应用受到严重副反应的限制,例如析氢反应和锌枝晶生长。在此,该研究提出了一种新的可生物降解电解质添加剂γ-戊内酯(GVL)的采用,仅添加1体积%(GVL与H 2 O的体积比)即可实现稳定的锌金属阳极。实验表征和理论计算相结合,验证了绿色GVL添加剂可以通过替换[Zn(H 2 O) 6 ] 2+中的H 2 O分子,竞争性地参与Zn 2+的溶剂化结构,从而有效降低反应活性水并抑制随后的副反应。此外,GVL分子优先吸附在Zn表面,以调节Zn的均匀沉积并抑制Zn枝晶的生长。因此,锌阳极表现出更高的稳定性、超长的循环寿命(超过 3500 小时)和高可逆性,库仑效率高达 99.69%。采用ZnSO 4 -GVL电解质的Zn||MnO 2全电池在0.5 A g -1下表现出219 mAh g -1的高容量,并且在550次循环后容量保持率提高到78%。这项工作为水系电池化学的生物基电解质添加剂提供了灵感,并促进了AZIBs的实际应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号