当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
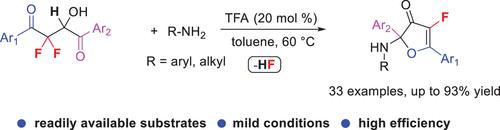
Synthesis of Fluoro 3(2H)-Furanones via a TFA-Catalyzed Dehydrofluorinative Cyclization of 2,2-Difluoro-3-hydroxy-1,4-diketones
Organic Letters ( IF 4.9 ) Pub Date : 2023-09-13 , DOI: 10.1021/acs.orglett.3c02765 Rongyao Li 1 , Jing Zhang 2 , Manman Sun 2 , Jinjing Xu 2 , Guo-Bo Huang 2 , Jianbo Yan 3 , Jianguo Yang 2 , Zhiming Wang 2 , Chao Ma 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-09-13 , DOI: 10.1021/acs.orglett.3c02765 Rongyao Li 1 , Jing Zhang 2 , Manman Sun 2 , Jinjing Xu 2 , Guo-Bo Huang 2 , Jianbo Yan 3 , Jianguo Yang 2 , Zhiming Wang 2 , Chao Ma 1
Affiliation
|
A TFA-catalyzed dehydrofluorinative cyclization of 2,2-difluoro-3-hydroxy-1,4-diketones has been developed in the presence of amines under mild conditions in which the difluorinated substrates are readily prepared according to our reported literature. This protocol provides a rapid construction of fluoro 3(2H)-furanones in good to excellent yields with good functional group tolerance. Easy scale-up synthesis also shows a practical advantage.
中文翻译:

通过 TFA 催化 2,2-二氟-3-羟基-1,4-二酮脱氟化氢环化合成氟 3(2H)-呋喃酮
根据我们报道的文献,在胺存在的温和条件下开发了 TFA 催化的 2,2-二氟-3-羟基-1,4-二酮的脱氟化氢环化,其中二氟化底物很容易制备。该方案提供了氟 3(2 H )-呋喃酮的快速构建,产率良好至优异,且具有良好的官能团耐受性。简单的放大合成也显示出实际优势。
更新日期:2023-09-13
中文翻译:

通过 TFA 催化 2,2-二氟-3-羟基-1,4-二酮脱氟化氢环化合成氟 3(2H)-呋喃酮
根据我们报道的文献,在胺存在的温和条件下开发了 TFA 催化的 2,2-二氟-3-羟基-1,4-二酮的脱氟化氢环化,其中二氟化底物很容易制备。该方案提供了氟 3(2 H )-呋喃酮的快速构建,产率良好至优异,且具有良好的官能团耐受性。简单的放大合成也显示出实际优势。














































 京公网安备 11010802027423号
京公网安备 11010802027423号