当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomically Dispersed Pt on CdS Nanosheets for Photocatalytic Evolution of H2 and 1,1-Diethoxyethane from Ethanol
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2023-09-13 , DOI: 10.1021/acsanm.3c03418 Huiqian Jiang 1 , Xiaohui Li 1 , Wenbin Zheng 1 , Hanshuai Xu 1 , Shuai Hou 2 , Lingxia Zheng 1 , Huajun Zheng 1 , Liang Mao 3 , Xiaowei Shi 1
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2023-09-13 , DOI: 10.1021/acsanm.3c03418 Huiqian Jiang 1 , Xiaohui Li 1 , Wenbin Zheng 1 , Hanshuai Xu 1 , Shuai Hou 2 , Lingxia Zheng 1 , Huajun Zheng 1 , Liang Mao 3 , Xiaowei Shi 1
Affiliation
|
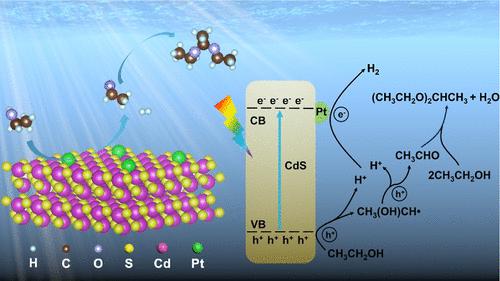
Efficient solar-to-H2 conversion coupled with high-value multicarbon chemical production is an appealing strategy to meet the demands in energy and environment; unfortunately, it remains a great challenge because of insufficient effective photocatalysts and vague catalytic mechanism. Here, Pt single sites are dispersed on a two-dimensional CdS nanosheet (PtSS-CdS) for simultaneous photocatalytic H2 and 1,1-diethoxyethane (DEE) evolution from ethanol. Structural characterizations elucidate that each Pt single atom is bonded with three surface S atoms in CdS, forming a Pt–S3 tetrahedron coordination structure. The strong semiconductor (CdS) and metal (Pt) interface interaction not only endows efficient migration of photoexcited electron from CdS to Pt single atom via Pt–S bonds but also promises Pt centers with optimized H adsorption energy, thus accelerating the H2 evolution reaction. Moreover, in situ electron spin resonance measurements reveal that the α-C–H bond rather than the O–H in ethanol prefers to be dehydrogenated by the surface holes of PtSS-CdS. Therefore, a remarkably promoted H2 generation rate (11.5 mmol g–1 h–1) is observed on PtSS-CdS under simulated solar light, and continuous H2 bubbles are generated over PtSS-CdS thin film upon light irradiation. Meanwhile, DEE is also formed with the selectivity of 97.9%. This study affords a promising strategy to develop high-performance catalysts toward photocatalytic H2 evolution and organic synthesis in a sustainable manner.
中文翻译:

CdS 纳米片上原子分散的 Pt 用于光催化从乙醇中演化出 H2 和 1,1-二乙氧基乙烷
高效的太阳能-H 2转化加上高价值的多碳化学品生产是满足能源和环境需求的一项有吸引力的策略;不幸的是,由于有效的光催化剂不足和催化机制不明确,这仍然是一个巨大的挑战。在这里,Pt 单位点分散在二维 CdS 纳米片 (Pt SS -CdS) 上,用于同时光催化从乙醇中释放 H 2和 1,1-二乙氧基乙烷 (DEE)。结构表征表明,每个 Pt 单原子与 CdS 中的三个表面 S 原子键合,形成 Pt-S 3四面体配位结构。半导体(CdS)和金属(Pt)的强界面相互作用不仅使光生电子通过Pt-S键从CdS有效迁移到Pt单原子,而且使Pt中心具有优化的H吸附能,从而加速H 2析出反应。此外,原位电子自旋共振测量表明,乙醇中的α-C-H键而不是O-H更容易被Pt SS -CdS的表面空穴脱氢。因此,在模拟太阳光下,在 Pt SS -CdS上观察到H 2生成速率显着提高(11.5 mmol g –1 h –1 ),并且连续 H 2在光照射下,Pt SS -CdS 薄膜上会产生气泡。同时,也形成了DEE,选择性为97.9%。这项研究为以可持续的方式开发用于光催化H 2析出和有机合成的高性能催化剂提供了一种有前途的策略。
更新日期:2023-09-13
中文翻译:

CdS 纳米片上原子分散的 Pt 用于光催化从乙醇中演化出 H2 和 1,1-二乙氧基乙烷
高效的太阳能-H 2转化加上高价值的多碳化学品生产是满足能源和环境需求的一项有吸引力的策略;不幸的是,由于有效的光催化剂不足和催化机制不明确,这仍然是一个巨大的挑战。在这里,Pt 单位点分散在二维 CdS 纳米片 (Pt SS -CdS) 上,用于同时光催化从乙醇中释放 H 2和 1,1-二乙氧基乙烷 (DEE)。结构表征表明,每个 Pt 单原子与 CdS 中的三个表面 S 原子键合,形成 Pt-S 3四面体配位结构。半导体(CdS)和金属(Pt)的强界面相互作用不仅使光生电子通过Pt-S键从CdS有效迁移到Pt单原子,而且使Pt中心具有优化的H吸附能,从而加速H 2析出反应。此外,原位电子自旋共振测量表明,乙醇中的α-C-H键而不是O-H更容易被Pt SS -CdS的表面空穴脱氢。因此,在模拟太阳光下,在 Pt SS -CdS上观察到H 2生成速率显着提高(11.5 mmol g –1 h –1 ),并且连续 H 2在光照射下,Pt SS -CdS 薄膜上会产生气泡。同时,也形成了DEE,选择性为97.9%。这项研究为以可持续的方式开发用于光催化H 2析出和有机合成的高性能催化剂提供了一种有前途的策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号