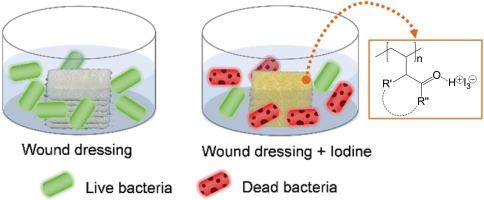
多重耐药 (MDR) 细菌会加剧伤口感染,因此需要具有抗菌特性的伤口敷料材料以防止慢性伤口的发展。碘 (I 2 ),通常为聚(乙烯基吡咯烷酮)-I 2 (PVP-I) 复合物,由于其功效、安全性和引起进一步耐药性的可能性较低,是伤口护理中广泛使用的防腐剂。尽管对 PVP-I 配方进行了广泛的研究,但仍有大量聚(乙烯基酰胺)可供选择,作为碘材料,特别是作为伤口敷料和医疗器械的涂层,这些材料尚未得到开发。因此,在本研究中,我们通过以下方法制备了一系列稳定的交联聚(乙烯基酰胺)涂层等离子聚合并研究了它们与 I 2的络合和释放的结构-性质关系。制备了等离子体聚合聚(N-乙烯基-2-吡咯烷酮)(pPVP)、聚(N-甲基-N-乙烯基乙酰胺)(pPMVA)和聚(N-乙烯基己内酰胺)(pPVCl)纳米级涂层,厚度均匀,为330-340纳米和低表面粗糙度。I 2作为聚碘化物的络合导致磷酸盐缓冲盐水(PBS)中的pPVCl涂层厚度显着增加,这与霍夫迈斯特效应一致。拉曼光谱证实 I 2仅以三碘化物离子 (I 3 -)在所有涂层中,这通过同步加速器X射线光电子能谱(XPS)实验得到证实。采用聚(乙烯基酰胺)的核磁共振(NMR)光谱进一步证明络合物质是三碘化氢(HI 3 )。I 2物质的负载量从 pPVP < pPMVA < pPVCl 增加,元素组成百分比分别为 1.3、1.8 和 2.9%,这与聚合和氧化过程中氮损失的程度相关,也与确定的负值一致。 zeta 电位值。然而,对 5 小时内释放的 I 2物质的定量表明,从 pPMVA (121.3 µg.cm -2 ) 和 pPVP (77.4 µg.cm -2 )中观察到最大释放量。) 和 pPVCl (74.6 µg.cm -2 ) 释放相似的量,突出显示聚(乙烯基酰胺)涂层中 I 2的释放是聚合物负载和组成的函数,而不仅仅是 I 2络合酰胺基序的函数。I 2复合 pPMVA 涂层伤口敷料对革兰氏阴性铜绿假单胞菌和革兰氏阳性金黄色葡萄球菌(与伤口感染相关的两种主要菌株)非常有效,可将细菌活力降低高达 4 和 7(log 10减少)数量级。总体而言,聚(乙烯基酰胺)涂层是用于预防和治疗伤口感染的有前途的碘伏。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Nanoscale iodophoric poly(vinyl amide) coatings for the complexation and release of iodine for antimicrobial surfaces
Wound infection exacerbated by multidrug-resistant (MDR) bacteria has necessitated the need for wound dressing materials with embedded antimicrobial property to prevent the development of chronic wounds. Iodine (I2), typically as a poly(vinyl pyrrolidone)-I2 (PVP–I) complex, is a widely used antiseptic agent in wound care owing to its efficacy, safety, and low probability to cause further resistance. Despite extensive research on PVP–I formulations, there is potentially a large selection of poly(vinyl amide)s that have remained largely unexplored as iodophoric materials, particularly as coatings for wound dressings and medical devices. Therefore, in this study we prepared a series of stable, cross-linked poly(vinyl amide) coatings via plasma polymerization and studied their structure–property relationships for the complexation and release of I2. Plasma polymerized poly(N-vinyl-2-pyrrolidone) (pPVP), poly(N-methyl-N-vinylacetamide) (pPMVA) and poly(N-vinylcaprolactam) (pPVCl) nanoscale coatings were prepared with uniform thicknesses of 330–340 nm and low surface roughness. Complexation of I2 as polyiodides resulted in a significant increase in the pPVCl coating thickness in phosphate buffered saline (PBS), consistent with the Hofmeister effect. Raman spectroscopy confirmed that the I2 was complexed exclusively as the triiodide ion (I3-) in all coatings, which was confirmed by synchrotron X-ray photoelectron spectroscopy (XPS) experiments. Nuclear magnetic resonance (NMR) spectroscopy of poly(vinyl amides) was employed to further prove that the complexing species was hydrogen triiodide (HI3). The loading of I2 species increased from pPVP < pPMVA < pPVCl, with percentage elemental compositions of 1.3, 1.8, and 2.9%, respectively, which correlated with the extent of nitrogen loss during polymerization and oxidation, and was also in agreement with determined negative zeta potential values. However, quantification of the released I2 species over 5 h showed that the greatest release was observed from pPMVA (121.3 µg.cm−2), with pPVP (77.4 µg.cm−2) and pPVCl (74.6 µg.cm−2) releasing a similar amount, highlighting that I2 release from poly(vinyl amide) coatings is a function of the loading and composition of the polymer, not just the I2 complexing amide motif. The I2 complexed pPMVA coated wound dressings were highly effective against Gram-negative Pseudomonas aeruginosa and Gram-positive Staphylococcus aureus, the two major strains associated with wound infection, reducing bacterial viability by up to 4 and 7 (log10 reduction) orders of magnitude. Overall, the poly(vinyl amide) coatings are promising iodophors for the prevention and treatment of wound infections.


































 京公网安备 11010802027423号
京公网安备 11010802027423号