当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of Some New Pyrazolo[1,5-a]pyrimidines and Evaluation of Their Antioxidant, Antibacterial (MIC and ZOI) Activities, and Cytotoxic Effect on MCF-7 Cell Lines
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-09-10 , DOI: 10.1002/cbdv.202301146 Mohammad Mehdi Vahedi 1 , Sakineh Asghari 1 , Mahmood Tajbakhsh 1 , Mojtaba Mohseni 2
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-09-10 , DOI: 10.1002/cbdv.202301146 Mohammad Mehdi Vahedi 1 , Sakineh Asghari 1 , Mahmood Tajbakhsh 1 , Mojtaba Mohseni 2
Affiliation
|
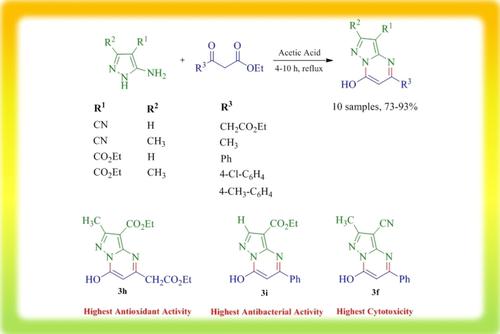
This study aims to synthesize some novel pyrazolo[1,5-a]pyrimidine derivatives, and investigate their biological activities. These compounds exhibited good to high antioxidant activities [2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capabilities]. Among them, Ethyl 5-(2-ethoxy-2-oxoethyl)-7-hydroxy-2-methylpyrazolo[1,5-a]pyrimidine-3-carboxylate (3h) showed the highest antioxidant activity [Half-maximal Inhibitory Concentration (IC50)=15.34 μM] compared to ascorbic acid (IC50=13.53 μM) as a standard compound. Their antibacterial activities were investigated against two Gram-positive bacteria (Bacillus subtilis, and Staphylococcus aureus) and two Gram-negative bacteria (Pseudomonas aeruginosa, and Escherichia coli). The results showed that Ethyl 7-hydroxy-5-phenylpyrazolo[1,5-a]pyrimidine-3-carboxylate (3i) has the best antibacterial activity against Gram-positive B. subtilis [Zone of Inhibition (ZOI)=23.0±1.4 mm, Minimum Inhibitory Concentration (MIC)=312 μM]. Also, the cytotoxicity of these compounds was assessed against breast cancer cell lines [human breast adenocarcinoma (MCF-7)], which 7-Hydroxy-2-methyl-5-phenylpyrazolo[1,5-a]pyrimidine-3-carbonitrile (3f) displayed the most cytotoxicity (IC50=55.97 μg/mL), in contrast with Lapatinib (IC50=79.38 μg/mL) as a known drug.
中文翻译:

一些新型吡唑并[1,5-a]嘧啶的制备及其抗氧化、抗菌(MIC和ZOI)活性以及对MCF-7细胞系的细胞毒作用的评价
本研究旨在合成一些新型吡唑并[1,5-a]嘧啶衍生物,并研究其生物活性。这些化合物表现出良好至高的抗氧化活性[2,2-二苯基-1-三硝基苯肼(DPPH)自由基清除能力]。其中,5-(2-乙氧基-2-氧代乙基)-7-羟基-2-甲基吡唑并[1,5-a]嘧啶-3-甲酸乙酯(3h)表现出最高的抗氧化活性[半最大抑制浓度(与作为标准化合物的抗坏血酸(IC 50 =13.53μM)相比,IC 50 ) = 15.34μM ] 。研究了它们对两种革兰氏阳性菌(枯草芽孢杆菌和金黄色葡萄球菌)和两种革兰氏阴性菌(铜绿假单胞菌和大肠杆菌)的抗菌活性。结果表明,7-羟基-5-苯基吡唑并[1,5-a]嘧啶-3-甲酸乙酯( 3i )对革兰氏阳性枯草芽孢杆菌的抗菌活性最好[抑菌圈(ZOI)=23.0±1.4] mm,最低抑菌浓度 (MIC)=312 μM]。此外,还评估了这些化合物对乳腺癌细胞系 [人乳腺癌 (MCF-7)] 的细胞毒性,其中 7-羟基-2-甲基-5-苯基吡唑并[1,5-a]嘧啶-3-甲腈((图3f )显示出最大的细胞毒性(IC 50 =55.97 μg/mL),与已知药物拉帕替尼(IC 50 =79.38 μg/mL)相反。
更新日期:2023-09-10
中文翻译:

一些新型吡唑并[1,5-a]嘧啶的制备及其抗氧化、抗菌(MIC和ZOI)活性以及对MCF-7细胞系的细胞毒作用的评价
本研究旨在合成一些新型吡唑并[1,5-a]嘧啶衍生物,并研究其生物活性。这些化合物表现出良好至高的抗氧化活性[2,2-二苯基-1-三硝基苯肼(DPPH)自由基清除能力]。其中,5-(2-乙氧基-2-氧代乙基)-7-羟基-2-甲基吡唑并[1,5-a]嘧啶-3-甲酸乙酯(3h)表现出最高的抗氧化活性[半最大抑制浓度(与作为标准化合物的抗坏血酸(IC 50 =13.53μM)相比,IC 50 ) = 15.34μM ] 。研究了它们对两种革兰氏阳性菌(枯草芽孢杆菌和金黄色葡萄球菌)和两种革兰氏阴性菌(铜绿假单胞菌和大肠杆菌)的抗菌活性。结果表明,7-羟基-5-苯基吡唑并[1,5-a]嘧啶-3-甲酸乙酯( 3i )对革兰氏阳性枯草芽孢杆菌的抗菌活性最好[抑菌圈(ZOI)=23.0±1.4] mm,最低抑菌浓度 (MIC)=312 μM]。此外,还评估了这些化合物对乳腺癌细胞系 [人乳腺癌 (MCF-7)] 的细胞毒性,其中 7-羟基-2-甲基-5-苯基吡唑并[1,5-a]嘧啶-3-甲腈((图3f )显示出最大的细胞毒性(IC 50 =55.97 μg/mL),与已知药物拉帕替尼(IC 50 =79.38 μg/mL)相反。






























 京公网安备 11010802027423号
京公网安备 11010802027423号