Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2023-09-10 , DOI: 10.1016/j.molstruc.2023.136620 Issam Ameziane El Hassani , Silvia A. Brandán , Salma Mortada , Suhana Arshad , E. Romano , Youssef Ramli , Joel T. Mague , My El Abbes Faouzi , Khalid Karrouchi , M'hammed Ansar
|
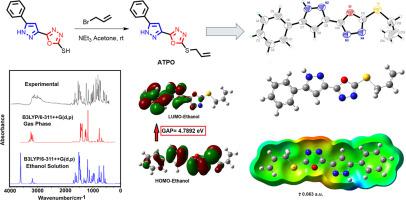
Here, we synthesized a new potent antidiabetic agent of pyrazolyl-1,3,4-oxadiazole derivative, named 2-(allylthio)-5-(5-phenyl-1H-pyrazol-3-yl)-1,3,4-oxadiazole (ATPO) and characterized it by FT-IR, UV-Vis, 1H NMR, 13C NMR, HRMS-ESI, X-ray crystal structure and evaluated as dual inhibitor for α-amylase and α-glucosidase enzymes. Predicted FT-IR, UV-Vis, 1H NMR, 13C NMR and UV-vis spectra and geometrical parameters for the optimized structures of ATPO in gas phase and ethanol solution by using B3LYP/6-311++G(d,p) method evidenced very good concordances with the corresponding experimental ones. Studies on atomic charges, molecular electrostatic potentials, stabilization energies and topological properties show that the phenyl, pyrazole and oxadiazole rings together with the allyl thiol moiety play a very important role in the stability, reactivity and behaviours of ATPO in both media. Thus, the compound is less stable in solution probably due to its higher reactivity in this medium and to its solvation energy (-75.89 kJ/mol). Complete vibrational assignments and the scaled force constants were performed for ATPO in both media. Also, ATPO exhibited outstanding α-glucosidase as well as α-amylase inhibitory potential with IC50 values of 35.47 ± 0.42 and 68.99 ± 1.08 µM, respectively, as compared to reference drug acarbose (IC50 (α-glucosidase) = 72.58 ± 0.68 µM, IC50 (α-amylase) = 115.6 ± 0.59 µM). In addition, ADMET studies were performed to investigate the possibility of using of the title compound as antidiabetic drug.
中文翻译:

2-(烯丙硫基)-5-(5-苯基-1H-吡唑-3-基)-1,3,4-恶二唑:合成、单晶 XRD、光谱表征、抗糖尿病活性、DFT 和 ADMET 研究
在这里,我们合成了一种新型有效的吡唑基-1,3,4-恶二唑衍生物抗糖尿病药物,命名为2-(烯丙基硫基)-5-(5-苯基-1 H-吡唑-3-基)-1,3,4 -恶二唑 ( ATPO ) 并通过 FT-IR、UV-Vis、 1 H NMR、13 C NMR、HRMS-ESI、X 射线晶体结构对其进行表征,并评估其作为 α-淀粉酶和 α-葡萄糖苷酶的双重抑制剂。预测 FT-IR、UV-Vis、1 H NMR、13 C NMR 和 UV-vis 光谱以及ATPO优化结构的几何参数使用B3LYP/6-311++G(d,p)方法在气相和乙醇溶液中的结果与相应的实验结果具有很好的一致性。对原子电荷、分子静电势、稳定能和拓扑性质的研究表明,苯基、吡唑和恶二唑环以及烯丙基硫醇部分对ATPO在两种介质中的稳定性、反应性和行为起着非常重要的作用。因此,该化合物在溶液中不太稳定,可能是由于其在该介质中的较高反应性及其溶剂化能(-75.89 kJ/mol)。在两种介质中对ATPO进行完整的振动分配和缩放力常数。另外,ATPO与参比药物阿卡波糖相比,表现出出色的 α-葡萄糖苷酶和 α-淀粉酶抑制潜力,IC 50值分别为 35.47 ± 0.42 和 68.99 ± 1.08 µM(IC 50 (α-葡萄糖苷酶) = 72.58 ± 0.68 µM,IC 50(α-淀粉酶) = 115.6 ± 0.59 µM)。此外,还进行了 ADMET 研究以调查标题化合物用作抗糖尿病药物的可能性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号