Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AMER1 deficiency promotes the distant metastasis of colorectal cancer by inhibiting SLC7A11- and FTL-mediated ferroptosis
Cell Reports ( IF 7.5 ) Pub Date : 2023-09-08 , DOI: 10.1016/j.celrep.2023.113110
Siqin Lei 1 , Chaoyi Chen 2 , Fengyan Han 1 , Jingwen Deng 1 , Dongdong Huang 3 , Lili Qian 4 , Ming Zhu 1 , Xiaohui Ma 5 , Maode Lai 6 , Enping Xu 6 , Honghe Zhang 7
Cell Reports ( IF 7.5 ) Pub Date : 2023-09-08 , DOI: 10.1016/j.celrep.2023.113110
Siqin Lei 1 , Chaoyi Chen 2 , Fengyan Han 1 , Jingwen Deng 1 , Dongdong Huang 3 , Lili Qian 4 , Ming Zhu 1 , Xiaohui Ma 5 , Maode Lai 6 , Enping Xu 6 , Honghe Zhang 7
Affiliation
|
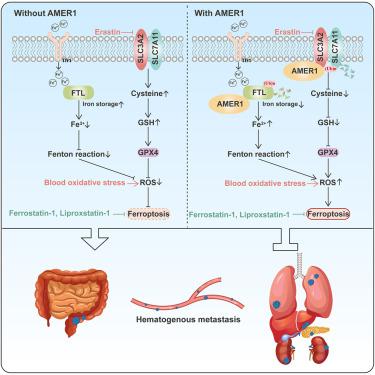
The crosstalk between ferroptosis and cancer metastasis remains unclear. Here, we identify AMER1 as a key regulator of ferroptosis. AMER1 loss causes resistance to ferroptosis in colorectal cancer (CRC) cells. Interestingly, AMER1-deficient CRC cells preferentially form distant metastases, while AMER1-naive CRC cells mainly invade lymph nodes. Moreover, the ferroptosis inhibitor liproxstatin-1 effectively promotes hematogenous transfer of AMER1-naive cells. Mechanistically, AMER1 binds to SLC7A11 and ferritin light chain (FTL) and recruits β-TrCP1/2, which degrade SLC7A11 and FTL by ubiquitination. Therefore, AMER1 deficiency increases cellular cystine levels but decreases the pool of labile free iron, thereby enhancing resistance to ferroptosis in CRC cells. Thus, AMER1 deficiency increases the survival of CRC cells in the blood under conditions of high oxidative stress and then promotes hematogenous metastasis of CRC. In conclusion, AMER1 mediates the crosstalk between ferroptosis and cancer metastasis, which provides a window of opportunity for treating metastatic colorectal cancer patients with AMER1 mutations.
中文翻译:

AMER1缺陷通过抑制SLC7A11和FTL介导的铁死亡促进结直肠癌的远处转移
铁死亡和癌症转移之间的相互作用仍不清楚。在这里,我们将 AMER1 确定为铁死亡的关键调节因子。AMER1 缺失会导致结直肠癌 (CRC) 细胞对铁死亡产生抵抗力。有趣的是,AMER1缺陷的CRC细胞优先形成远处转移,而AMER1幼稚的CRC细胞主要侵入淋巴结。此外,铁死亡抑制剂 liproxstatin-1 能有效促进 AMER1 初始细胞的血行转移。从机制上讲,AMER1 与 SLC7A11 和铁蛋白轻链 (FTL) 结合并招募 β-TrCP1/2,后者通过泛素化降解 SLC7A11 和 FTL。因此,AMER1 缺乏会增加细胞胱氨酸水平,但会减少不稳定游离铁的数量,从而增强 CRC 细胞对铁死亡的抵抗力。因此,AMER1缺陷会增加血液中CRC细胞在高氧化应激条件下的存活率,进而促进CRC的血行转移。总之,AMER1介导铁死亡和癌症转移之间的串扰,这为治疗具有AMER1突变的转移性结直肠癌患者提供了机会之窗。
更新日期:2023-09-08
中文翻译:

AMER1缺陷通过抑制SLC7A11和FTL介导的铁死亡促进结直肠癌的远处转移
铁死亡和癌症转移之间的相互作用仍不清楚。在这里,我们将 AMER1 确定为铁死亡的关键调节因子。AMER1 缺失会导致结直肠癌 (CRC) 细胞对铁死亡产生抵抗力。有趣的是,AMER1缺陷的CRC细胞优先形成远处转移,而AMER1幼稚的CRC细胞主要侵入淋巴结。此外,铁死亡抑制剂 liproxstatin-1 能有效促进 AMER1 初始细胞的血行转移。从机制上讲,AMER1 与 SLC7A11 和铁蛋白轻链 (FTL) 结合并招募 β-TrCP1/2,后者通过泛素化降解 SLC7A11 和 FTL。因此,AMER1 缺乏会增加细胞胱氨酸水平,但会减少不稳定游离铁的数量,从而增强 CRC 细胞对铁死亡的抵抗力。因此,AMER1缺陷会增加血液中CRC细胞在高氧化应激条件下的存活率,进而促进CRC的血行转移。总之,AMER1介导铁死亡和癌症转移之间的串扰,这为治疗具有AMER1突变的转移性结直肠癌患者提供了机会之窗。

































 京公网安备 11010802027423号
京公网安备 11010802027423号