当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Total Synthesis of (−)-Zygadenine
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-09-08 , DOI: 10.1021/jacs.3c08039 Yinliang Guo 1 , Jia-Tian Lu 1, 2 , Runting Fang 3 , Yang Jiao 1 , Jiaqi Liu 1 , Tuoping Luo 1, 2, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-09-08 , DOI: 10.1021/jacs.3c08039 Yinliang Guo 1 , Jia-Tian Lu 1, 2 , Runting Fang 3 , Yang Jiao 1 , Jiaqi Liu 1 , Tuoping Luo 1, 2, 3
Affiliation
|
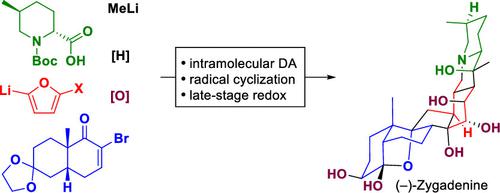
The Veratrum alkaloids are highly complex steroidal alkaloids characterized by their intricate structural and stereochemical features and exhibit a diverse range of pharmacological activities. A new synthetic pathway has been developed to access this family of natural products, which enabled the first total synthesis of (−)-zygadenine. This synthetic route entails the construction of a hexacyclic carbon skeleton through a stereoselective intramolecular Diels–Alder reaction, followed by a radical cyclization. Subsequently, a meticulously designed sequence of redox manipulations was optimized to achieve the de novo synthesis of this highly oxidized Veratrum alkaloid.
中文翻译:

(−)-Zygadenine 的对映选择性全合成
藜芦生物碱是高度复杂的甾体生物碱,具有复杂的结构和立体化学特征,并表现出多种药理活性。已经开发出一种新的合成途径来获取该天然产物家族,从而首次实现了 (−)-zygadenine 的全合成。该合成路线需要通过立体选择性分子内狄尔斯-阿尔德反应构建六环碳骨架,然后进行自由基环化。随后,优化了精心设计的氧化还原操作序列,以实现这种高度氧化的藜芦生物碱的从头合成。
更新日期:2023-09-08
中文翻译:

(−)-Zygadenine 的对映选择性全合成
藜芦生物碱是高度复杂的甾体生物碱,具有复杂的结构和立体化学特征,并表现出多种药理活性。已经开发出一种新的合成途径来获取该天然产物家族,从而首次实现了 (−)-zygadenine 的全合成。该合成路线需要通过立体选择性分子内狄尔斯-阿尔德反应构建六环碳骨架,然后进行自由基环化。随后,优化了精心设计的氧化还原操作序列,以实现这种高度氧化的藜芦生物碱的从头合成。







































 京公网安备 11010802027423号
京公网安备 11010802027423号