Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2023-09-06 , DOI: 10.1016/j.jclepro.2023.138698 Hao Qi , Qirong Yang , Xinru Ma , Mengyu Wan , Zijun Zhang , Haoxi Ben , Lianghuan Wei , Zhaoying Li
|
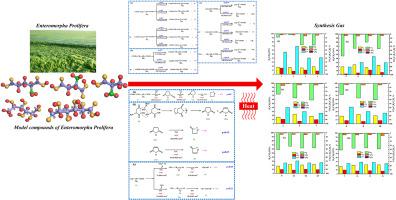
The large-scale flooding of Enteromorpha prolifera (EP) forms “green tide”, which causes severe environmental problems. However, EP, an algal biomass, can be converted into syngas via pyrolysis. In this study, the pyrolysis mechanisms of the main compositional models of EP used to produce syngas are investigated using a combination of molecular dynamics simulations and fixed-bed experiments. Rhamnose (Rha), glucuronic acid (GlcA), aspartic acid (Asp), glutamic acid (Glu), and alanine (Ala) are used as the model compounds. The results indicate that H2 is primarily produced by Glu (reached 24.68 vol%), followed by Asp and Ala, and CO is produced by Ala (reached 44.94 vol%), Glu, and Asp. The molecular dynamics simulation results indicate that the energy barriers for the pyrolysis of Glu and Ala to produce H2 and CO, respectively, are the lowest. Three amino acids undergo dehydration, deamination, and decarboxylation. Moreover, the synergistic effect of the three amino acids in co-pyrolysis is reflected in the production of hydrogen groups from Asp; and the hydrogen group then attacks Glu to seize the hydrogen group on it to produce hydrogen. In addition, the H2 content in the syngas produced by the separate pyrolysis of Rha (17.75 vol%) and GlcA (17.35 vol%) are similar and lower than those of the Rha and GlcA mixtures (19.12 vol%). The CO content in the pyrolysis gas of GlcA is higher than that of Rha. The simulation results prove that the amino acids undergo dicarboxylic and deamination reactions to produce amino acids and CO2. Meanwhile, polysaccharide sulfate breaks glycosidic bonds, producing free radicals such as hydrogen and hydroxyl groups at 700 K. When the temperature rises to 1900 K, the products of the previous stage further break the bond, producing small-molecule gases. However, the addition of polysaccharide model compounds promotes the production of CO2 and C O groups and inhibits the formation of CO. Co-pyrolysis cannot increase H2, and the synergistic effect is not significant. Moreover, Ni and SAPO-34 exhibited good catalytic activities, which could increase syngas production. A raw materials to catalyst ratio of 5:1 is a good choice because it improves the H2 and CO contents and makes economical use of Ni. With the increase of Ni catalyst cycles, H2 content also shows a decreasing trend, from 22.86 vol% to 16.27 vol% (third cycle). With the number of SAPO-34 cycles, the increase of CO content, from 28.66 vol% to 32.67 vol% is explained by the increase in the specific surface area of the catalyst caused by repeated use, which increases the selectivity of C2 and enhances the ability of decarbonylation. This study focuses on the syngas reaction paths and pyrolysis reactions of different EP model compounds. The pyrolysis mechanism of EP to produce syngas is determined using a combination of experiments and simulations, providing a method for the energy utilization of EP.
O groups and inhibits the formation of CO. Co-pyrolysis cannot increase H2, and the synergistic effect is not significant. Moreover, Ni and SAPO-34 exhibited good catalytic activities, which could increase syngas production. A raw materials to catalyst ratio of 5:1 is a good choice because it improves the H2 and CO contents and makes economical use of Ni. With the increase of Ni catalyst cycles, H2 content also shows a decreasing trend, from 22.86 vol% to 16.27 vol% (third cycle). With the number of SAPO-34 cycles, the increase of CO content, from 28.66 vol% to 32.67 vol% is explained by the increase in the specific surface area of the catalyst caused by repeated use, which increases the selectivity of C2 and enhances the ability of decarbonylation. This study focuses on the syngas reaction paths and pyrolysis reactions of different EP model compounds. The pyrolysis mechanism of EP to produce syngas is determined using a combination of experiments and simulations, providing a method for the energy utilization of EP.
中文翻译:

浒苔主要模型化合物热解产生合成气的机制
浒苔大规模泛滥形成“绿潮”,造成严重的环境问题。然而,EP(一种藻类生物质)可以通过热解转化为合成气。在本研究中,结合分子动力学模拟和固定床实验,研究了用于生产合成气的EP主要成分模型的热解机制。使用鼠李糖(Rha)、葡萄糖醛酸(GlcA)、天冬氨酸(Asp)、谷氨酸(Glu)和丙氨酸(Ala)作为模型化合物。结果表明H 2主要由Glu(达到24.68 vol%)产生,其次是Asp和Ala,CO由Ala(达到44.94 vol%)、Glu和Asp产生。分子动力学模拟结果表明,Glu和Ala热解分别产生H 2和CO的能垒最低。三种氨基酸经历脱水、脱氨和脱羧。此外,三种氨基酸共热解的协同效应体现在Asp产生氢基团上;然后氢基团攻击Glu,夺取其上的氢基团产生氢气。此外,H 2Rha (17.75 vol%) 和 GlcA (17.35 vol%) 单独热解产生的合成气中的含量相似,并且低于 Rha 和 GlcA 混合物 (19.12 vol%) 的含量。GlcA热解气中CO含量高于Rha。模拟结果证明氨基酸发生二羧基和脱氨基反应,生成氨基酸和CO 2 。同时,硫酸多糖在700 K时断裂糖苷键,产生氢、羟基等自由基。当温度升至1900 K时,前一阶段的产物进一步断裂糖苷键,产生小分子气体。然而,多糖模型化合物的添加促进了CO 2和C的产生 O基团并抑制CO的形成。共热解不能增加H 2 ,协同效应不显着。此外,Ni和SAPO-34表现出良好的催化活性,可以提高合成气产量。原料与催化剂的比例为5:1是一个不错的选择,因为它提高了H 2和CO的含量并经济地利用了Ni。随着Ni催化剂循环次数的增加,H 2含量也呈现下降趋势,从 22.86 vol% 降至 16.27 vol%(第三个周期)。随着SAPO-34循环次数的增加,CO含量从28.66 vol%增加到32.67 vol%,这可以解释为重复使用导致催化剂比表面积增加,这增加了C2的选择性并增强了脱羰能力。本研究重点研究不同 EP 模型化合物的合成气反应路径和热解反应。通过实验和模拟相结合的方法,确定了EP热解生产合成气的机理,为EP的能源利用提供了方法。
O基团并抑制CO的形成。共热解不能增加H 2 ,协同效应不显着。此外,Ni和SAPO-34表现出良好的催化活性,可以提高合成气产量。原料与催化剂的比例为5:1是一个不错的选择,因为它提高了H 2和CO的含量并经济地利用了Ni。随着Ni催化剂循环次数的增加,H 2含量也呈现下降趋势,从 22.86 vol% 降至 16.27 vol%(第三个周期)。随着SAPO-34循环次数的增加,CO含量从28.66 vol%增加到32.67 vol%,这可以解释为重复使用导致催化剂比表面积增加,这增加了C2的选择性并增强了脱羰能力。本研究重点研究不同 EP 模型化合物的合成气反应路径和热解反应。通过实验和模拟相结合的方法,确定了EP热解生产合成气的机理,为EP的能源利用提供了方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号