当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Purine DNA Constructs Designed to Expand the Genetic Code: Functionalization, Impact of Ionic Forms, and Molecular Recognition of 7-Deazaxanthine–7-Deazapurine-2,6-diamine Base Pairs and Their Purine Counterparts
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-09-05 , DOI: 10.1021/acs.joc.3c01370 Somnath Shivaji Chandankar 1 , Dasharath Kondhare 1 , Peter Leonard 1 , Frank Seela 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-09-05 , DOI: 10.1021/acs.joc.3c01370 Somnath Shivaji Chandankar 1 , Dasharath Kondhare 1 , Peter Leonard 1 , Frank Seela 1, 2
Affiliation
|
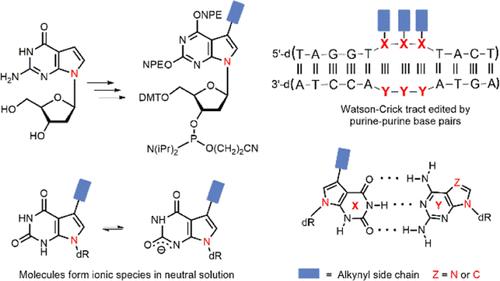
Purine DNA represents an alternative pairing system formed by two purines in the base pair with the recognition elements of Watson–Crick DNA. Base functionalization of 7-deaza-2′-deoxyxanthosine with ethynyl and octadiynyl residues led to clickable side chain derivatives with short and long linker arms. As complementary bases, purine-2,6-diamine or 7-deazapurine-2,6-diamine 2′-deoxyribonucleosides were used. 7-Deaza-7-iodo-2′-deoxyxanthosine served as a starting material for Sonogashira cross-coupling and the p-nitrophenylethyl group for base protection. Phosphoramidite building blocks for DNA synthesis were prepared. Oligonucleotides containing single modifications or runs of three purine base pairs embedded in 12-mer Watson–Crick DNA were synthesized and hybridized with complementary strands with purine- or 7-deazapurine-2,6-diamine located opposite to the xanthine derivatives. The stability of base pairs was evaluated in a comparative study on the basis of DNA melting experiments and Tm values. As 7-deazaxanthine and xanthine nucleosides form anionic forms at neutral pH, duplex stability became pK-dependent, and the system with 7-deazapurine displayed a significant higher stability as that containing xanthine. Alkynyl side chains are well accommodated in the purine–purine helix. Click adducts with pyrene showed that short linker arms destabilize duplexes, whereas long linkers increase duplex stability. CD and fluorescence measurements provide further insights into purine–purine base pairing.
中文翻译:

旨在扩展遗传密码的嘌呤 DNA 构建体:7-脱氮黄嘌呤–7-脱氮嘌呤-2,6-二胺碱基对及其嘌呤对应物的功能化、离子形式的影响和分子识别
嘌呤DNA代表由碱基对中的两个嘌呤与沃森-克里克DNA的识别元件形成的替代配对系统。带有乙炔基和辛二炔基残基的 7-deaza-2'-脱氧黄嘌呤核苷的碱基官能化产生了具有短和长连接臂的可点击侧链衍生物。使用嘌呤-2,6-二胺或7-脱氮嘌呤-2,6-二胺2'-脱氧核糖核苷作为互补碱基。7-Deaza-7-iodo-2'-deoxyxanthosine 用作 Sonogashira 交叉偶联的起始材料,并且对硝基苯乙基用作碱基保护。制备了用于 DNA 合成的亚磷酰胺结构单元。合成了包含单个修饰或嵌入 12 聚体 Watson-Crick DNA 中的三个嘌呤碱基对的寡核苷酸,并与位于黄嘌呤衍生物对面的嘌呤-或 7-脱氮嘌呤-2,6-二胺的互补链杂交。基于 DNA 熔解实验和T m值的比较研究评估了碱基对的稳定性。由于7-脱氮杂嘌呤和黄嘌呤核苷在中性pH下形成阴离子形式,双链体稳定性变得p K依赖性,并且具有7-脱氮嘌呤的系统表现出比含有黄嘌呤的系统显着更高的稳定性。炔基侧链很好地容纳在嘌呤-嘌呤螺旋中。与芘的点击加合物表明,短连接臂使双链体不稳定,而长连接臂则增加双链体稳定性。CD 和荧光测量提供了对嘌呤-嘌呤碱基配对的进一步了解。
更新日期:2023-09-05
中文翻译:

旨在扩展遗传密码的嘌呤 DNA 构建体:7-脱氮黄嘌呤–7-脱氮嘌呤-2,6-二胺碱基对及其嘌呤对应物的功能化、离子形式的影响和分子识别
嘌呤DNA代表由碱基对中的两个嘌呤与沃森-克里克DNA的识别元件形成的替代配对系统。带有乙炔基和辛二炔基残基的 7-deaza-2'-脱氧黄嘌呤核苷的碱基官能化产生了具有短和长连接臂的可点击侧链衍生物。使用嘌呤-2,6-二胺或7-脱氮嘌呤-2,6-二胺2'-脱氧核糖核苷作为互补碱基。7-Deaza-7-iodo-2'-deoxyxanthosine 用作 Sonogashira 交叉偶联的起始材料,并且对硝基苯乙基用作碱基保护。制备了用于 DNA 合成的亚磷酰胺结构单元。合成了包含单个修饰或嵌入 12 聚体 Watson-Crick DNA 中的三个嘌呤碱基对的寡核苷酸,并与位于黄嘌呤衍生物对面的嘌呤-或 7-脱氮嘌呤-2,6-二胺的互补链杂交。基于 DNA 熔解实验和T m值的比较研究评估了碱基对的稳定性。由于7-脱氮杂嘌呤和黄嘌呤核苷在中性pH下形成阴离子形式,双链体稳定性变得p K依赖性,并且具有7-脱氮嘌呤的系统表现出比含有黄嘌呤的系统显着更高的稳定性。炔基侧链很好地容纳在嘌呤-嘌呤螺旋中。与芘的点击加合物表明,短连接臂使双链体不稳定,而长连接臂则增加双链体稳定性。CD 和荧光测量提供了对嘌呤-嘌呤碱基配对的进一步了解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号