当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Continuous Flow Processes to Access Pyrrolo[2,1-f][1,2,4]triazin-4-amine: An RSM for the Synthesis of Antiviral Drugs
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-09-06 , DOI: 10.1021/acs.oprd.3c00184 Maksim Vasilev 1 , Aravind S. Gangu 1 , Praveen Gajula 1 , Nathaniel D. Kaetzel 1 , Vasudevan Natarajan 1 , Bimbisar Desai 1 , Gopal Sirasani 1 , Bo Qu 1 , Chris H. Senanayake 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-09-06 , DOI: 10.1021/acs.oprd.3c00184 Maksim Vasilev 1 , Aravind S. Gangu 1 , Praveen Gajula 1 , Nathaniel D. Kaetzel 1 , Vasudevan Natarajan 1 , Bimbisar Desai 1 , Gopal Sirasani 1 , Bo Qu 1 , Chris H. Senanayake 1
Affiliation
|
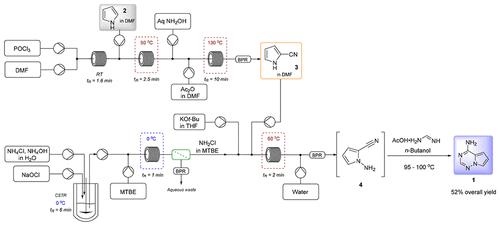
Pyrrolo[2,1-f][1,2,4]triazines are important scaffolds in a number of active pharmaceutical ingredients with a broad range of biological activities to treat broad-spectrum viral infections. We recently reported the synthesis at the kilogram scale in batch mode with extensive process safety studies, where NaH was applied as the base to deprotonate 2-cyanopyrrole, and then in situ prepared monochloramine solution was utilized for the N-amination, followed by cyclization with formamidine acetate to produce the required pyrrolo[2,1-f][1,2,4]triazine (1) as the regulatory starting material for the antiviral drug Remdesivir. To meet the market demand of Remdesivir for the treatment of the recently emerged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a high-throughput process is required to supply this key starting material on a large scale in a timely manner. In this Article, we report the second-generation synthesis of pyrrolo[2,1-f][1,2,4]triazine 1 by employing continuous flow chemistry tools. The amination step was adapted to continuous flow by applying in situ monochloramine synthesis and utilizing a process-friendly soluble base KOt-Bu. The new multistage continuous flow approaches, including both chemical steps, extractions and separations, afford a viable process to access this widely employed key starting material 1 for the synthesis of Remdesivir.
中文翻译:

开发获取吡咯并[2,1-f][1,2,4]三嗪-4-胺的连续流程:用于合成抗病毒药物的 RSM
吡咯并[2,1- f ][1,2,4]三嗪是许多活性药物成分的重要支架,具有广泛的生物活性,可治疗广谱病毒感染。我们最近报道了以批量模式进行的公斤级合成,并进行了广泛的工艺安全研究,其中使用 NaH 作为碱使 2-氰基吡咯去质子化,然后使用原位制备的一氯胺溶液进行N -胺化,然后用乙酸甲脒生产所需的吡咯并[2,1- f ][1,2,4]三嗪 ( 1 ),作为抗病毒药物瑞德西韦的监管起始原料。为了满足瑞德西韦治疗最近出现的严重急性呼吸综合征冠状病毒2(SARS-CoV-2)的市场需求,需要高通量工艺来及时大规模供应这一关键原材料。在本文中,我们报道了采用连续流化学工具第二代吡咯并[2,1- f ][1,2,4]三嗪1的合成。通过应用原位一氯胺合成并利用工艺友好的可溶性碱 KO t -Bu,使胺化步骤适应连续流。新的多级连续流动方法,包括化学步骤、萃取和分离,提供了一种可行的工艺来获取这种广泛使用的合成瑞德西韦的关键原材料1 。
更新日期:2023-09-06
中文翻译:

开发获取吡咯并[2,1-f][1,2,4]三嗪-4-胺的连续流程:用于合成抗病毒药物的 RSM
吡咯并[2,1- f ][1,2,4]三嗪是许多活性药物成分的重要支架,具有广泛的生物活性,可治疗广谱病毒感染。我们最近报道了以批量模式进行的公斤级合成,并进行了广泛的工艺安全研究,其中使用 NaH 作为碱使 2-氰基吡咯去质子化,然后使用原位制备的一氯胺溶液进行N -胺化,然后用乙酸甲脒生产所需的吡咯并[2,1- f ][1,2,4]三嗪 ( 1 ),作为抗病毒药物瑞德西韦的监管起始原料。为了满足瑞德西韦治疗最近出现的严重急性呼吸综合征冠状病毒2(SARS-CoV-2)的市场需求,需要高通量工艺来及时大规模供应这一关键原材料。在本文中,我们报道了采用连续流化学工具第二代吡咯并[2,1- f ][1,2,4]三嗪1的合成。通过应用原位一氯胺合成并利用工艺友好的可溶性碱 KO t -Bu,使胺化步骤适应连续流。新的多级连续流动方法,包括化学步骤、萃取和分离,提供了一种可行的工艺来获取这种广泛使用的合成瑞德西韦的关键原材料1 。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号