Results in Chemistry ( IF 2.5 ) Pub Date : 2023-09-04 , DOI: 10.1016/j.rechem.2023.101093 Keshav B. Gangurde , Vishnu A. Adole , Dattatraya S. Ghotekar
|
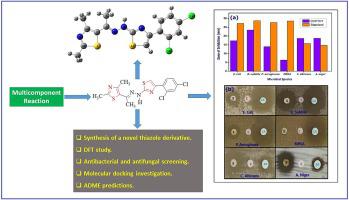
In this paper, we describe the synthesis of a novel (E)-5-(1-(2-(4-(2,4-dichlorophenyl)thiazol-2-yl)hydrazineylidene)ethyl)-2,4-dimethylthiazole (DCPTHT) via multicomponent reaction between 1-(2,4-dimethylthiazol-5-yl)ethan-1-one, thiosemicarbazide and 2-bromo-1-(2,4-dichlorophenyl)ethan-1-one. The structure of DCPTHT was confirmed on the basis of FT-IR, 1HNMR and 13C NMR characterizations. The molecular structure of the novel thiazole derivative was examined using density functional theory (DFT) simulations at the B3LYP/6-311G (d,p) level of theory. Molecular simulations were made for total energy, HOMO and LUMO energy, and Mulliken atomic charges. In a dimethyl sulfoxide solvent, the electronic absorption spectra were acquired, and TD-DFT calculations were used to discuss the band assignments. By correlating the experimental and simulated spectra, NMR assignments and interpretations were also established. Remarkably, antibacterial and antifungal screening was used to examine the biological profile of DCPTHT. Antibacterial screening was performed against E. coli, B. subtilis, P. aeruginosa, and MRSA while the antifungal screening was performed against A. niger and C. albicans. It was found that the newly synthesized thiazole derivative demonstrated potent antifungal activity on the investigated fungal species. Molecular docking study against cytochrome P450 14α-sterol demethylase (CYP51) (PDB id: 5v5z). Furthermore, ADME predictions are also discussed. The molecular docking studies revealed mostly hydrophobic and van der Waals interactions with different amino acid residues.
中文翻译:

计算研究:新型 (E)-5-(1-(2-(4-(2,4-二氯苯基)) 的合成、光谱(UV-vis、IR、NMR)、抗菌、抗真菌、抗氧化剂、分子对接和 ADME噻唑-2-基)亚肼基)乙基)-2,4-二甲基噻唑
在本文中,我们描述了新型( E )-5-(1-(2-(4-(2,4-二氯苯基)噻唑-2-基)亚肼基)乙基)-2,4-二甲基噻唑( DCPTHT)通过1-(2,4-二甲基噻唑-5-基)乙烷-1-酮、氨基硫脲和2-溴-1-(2,4-二氯苯基)乙烷-1-酮之间的多组分反应。DCPTHT的结构通过FT-IR、1 HNMR和1313C NMR 表征。使用 B3LYP/6-311G (d,p) 理论水平的密度泛函理论 (DFT) 模拟检查了新型噻唑衍生物的分子结构。对总能量、HOMO 和 LUMO 能量以及 Mulliken 原子电荷进行了分子模拟。在二甲亚砜溶剂中,获得电子吸收光谱,并使用 TD-DFT 计算来讨论能带分配。通过关联实验和模拟光谱,还建立了 NMR 分配和解释。值得注意的是,使用抗菌和抗真菌筛选来检查 DCPTHT 的生物学特征。对大肠杆菌、枯草芽孢杆菌、铜绿假单胞菌和耐甲氧西林金黄色葡萄球菌进行抗菌筛选同时针对A. niger和C进行了抗真菌筛选。白色念珠菌。结果发现,新合成的噻唑衍生物对所研究的真菌物种表现出有效的抗真菌活性。针对细胞色素 P450 14α-甾醇脱甲基酶 (CYP51) 的分子对接研究(PDB id:5v5z)。此外,还讨论了 ADME 预测。分子对接研究主要揭示了与不同氨基酸残基的疏水性和范德华相互作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号