当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ti2+ Site-Promoted N≡N Bond Activation in LaTiO3–x Nanosheets for Nitrogen Photofixation
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-09-06 , DOI: 10.1021/acscatal.3c02198 Mengyang Xia 1 , Ben Chong 1 , Xiangjiao Gong 1 , Hang Xiao 1 , He Li 1 , Honghui Ou 1 , Bin Zhang 1 , Guidong Yang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-09-06 , DOI: 10.1021/acscatal.3c02198 Mengyang Xia 1 , Ben Chong 1 , Xiangjiao Gong 1 , Hang Xiao 1 , He Li 1 , Honghui Ou 1 , Bin Zhang 1 , Guidong Yang 1
Affiliation
|
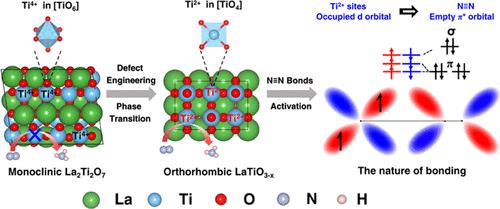
Using solar energy to fix N2 and produce NH3 is a promising route. For nitrogen photofixation, the transition-metal active site in the low oxidation state is conducive to the adsorption of N2, but it is often difficult to further dissociate N2, restricting the reaction. Therefore, it is necessary to precisely modulate the electron energy level of the active site to couple with the molecular orbitals of N2, thus reducing the energy barrier of N2 dissociation. Here, the perovskite-type LaTiO3–x with an ultralow oxidation state Ti2+ site is achieved via in situ modulation of phase transition and defect engineering. The obtained Ti2+ sites could inject more d-orbital electrons into the N2 π* antibonding orbitals to achieve N2 activation and dissociation. Therefore, compared with pristine La2Ti2O7 and La2Ti2O7–x samples without ammonia production activity, LaTiO3–x samples showed a remarkable performance for photocatalytic N2 fixation. The NH3 generation rate reached up to 107 μmol gcat–1 h–1 after the 1st hour, and the average NH3 generation rate after 4 h was approximately 51.5 μmol gcat–1 h–1. Furthermore, in situ characterization and density functional theory (DFT) calculations revealed the role of Ti sites with different oxidation states (Ti4+, Ti3+, Ti2+) in N2 activation, which would provide a unique perspective for designing efficient N2 fixation catalysts.
中文翻译:

Ti2+ 位点促进 LaTiO3–x 纳米片中 N=N 键的活化,用于氮光固定
利用太阳能固定N 2并生产NH 3是一条很有前途的途径。对于氮光固定,低氧化态的过渡金属活性位点有利于N 2的吸附,但往往难以进一步解离N 2,限制了反应。因此,需要精确调节活性位点的电子能级以与N 2分子轨道耦合,从而降低N 2解离的能垒。此处,具有超低氧化态 Ti 2+的钙钛矿型 LaTiO 3– x该位点是通过相变和缺陷工程的原位调制来实现的。所获得的Ti 2+位点可以将更多的d轨道电子注入到N 2 π*反键轨道中,从而实现N 2 的活化和解离。因此,与没有产氨活性的原始La 2 Ti 2 O 7和La 2 Ti 2 O 7– x样品相比,LaTiO 3– x样品表现出显着的光催化N 2固定性能。NH 3生成率达到107 μmol g cat –1 h第一小时后NH 3生成速率约为51.5 μmol g cat –1 h –1,4小时后平均NH 3生成速率约为51.5 μmol g cat –1 h –1。此外,原位表征和密度泛函理论(DFT)计算揭示了不同氧化态(Ti 4+、Ti 3+、Ti 2+)的Ti位点在N 2活化中的作用,这将为设计高效的Ti位点提供独特的视角。 N 2固定催化剂。
更新日期:2023-09-06
中文翻译:

Ti2+ 位点促进 LaTiO3–x 纳米片中 N=N 键的活化,用于氮光固定
利用太阳能固定N 2并生产NH 3是一条很有前途的途径。对于氮光固定,低氧化态的过渡金属活性位点有利于N 2的吸附,但往往难以进一步解离N 2,限制了反应。因此,需要精确调节活性位点的电子能级以与N 2分子轨道耦合,从而降低N 2解离的能垒。此处,具有超低氧化态 Ti 2+的钙钛矿型 LaTiO 3– x该位点是通过相变和缺陷工程的原位调制来实现的。所获得的Ti 2+位点可以将更多的d轨道电子注入到N 2 π*反键轨道中,从而实现N 2 的活化和解离。因此,与没有产氨活性的原始La 2 Ti 2 O 7和La 2 Ti 2 O 7– x样品相比,LaTiO 3– x样品表现出显着的光催化N 2固定性能。NH 3生成率达到107 μmol g cat –1 h第一小时后NH 3生成速率约为51.5 μmol g cat –1 h –1,4小时后平均NH 3生成速率约为51.5 μmol g cat –1 h –1。此外,原位表征和密度泛函理论(DFT)计算揭示了不同氧化态(Ti 4+、Ti 3+、Ti 2+)的Ti位点在N 2活化中的作用,这将为设计高效的Ti位点提供独特的视角。 N 2固定催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号