当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Scalable, Chromatography-Free Synthesis of 2-(3-Bromophenyl)-2H-1,2,3-triazole through N−N Bond Forming Cyclization
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2023-09-05 , DOI: 10.1002/hlca.202300146 Philipp Kohler 1 , Ivan Schindelholz 2 , Gabriel Schäfer 2
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2023-09-05 , DOI: 10.1002/hlca.202300146 Philipp Kohler 1 , Ivan Schindelholz 2 , Gabriel Schäfer 2
Affiliation
|
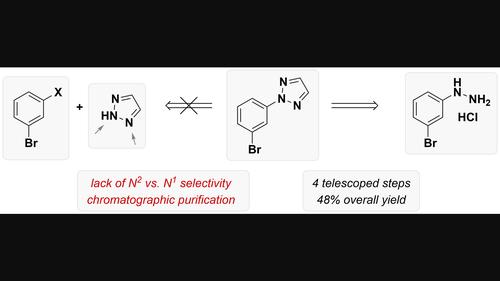
2-(3-Bromophenyl)-2H-1,2,3-triazole is a low molecular-weight building block that is not readily accessible in pure form by standard synthetic approaches due to competing formation of the triazol-1-yl regioisomer. After investigation of a wide range of synthetic routes, a process based on construction of the triazole heterocycle by cyclization of an activated dihydrazone was developed. A suitable isolation method had to be identified before embarking on a demonstration run on >100 g scale without the need for chromatography. The new process delivered the desired building block in 48 % overall yield over four telescoped steps with excellent purity (99 % a/a).
中文翻译:

通过 N−N 键形成环化可扩展、无需色谱法合成 2-(3-溴苯基)-2H-1,2,3-三唑
2-(3-溴苯基)-2 H -1,2,3-三唑是一种低分子量结构单元,由于三唑-1-基区域异构体的竞争形成,不易通过标准合成方法以纯形式获得。在研究了多种合成路线后,开发了一种基于通过活化二腙环化构建三唑杂环的方法。在开始 >100 g 规模的演示运行(无需色谱法)之前,必须确定合适的分离方法。新工艺通过四个叠接步骤以 48% 的总收率交付了所需的构建模块,并具有出色的纯度 (99% a/a)。
更新日期:2023-09-05
中文翻译:

通过 N−N 键形成环化可扩展、无需色谱法合成 2-(3-溴苯基)-2H-1,2,3-三唑
2-(3-溴苯基)-2 H -1,2,3-三唑是一种低分子量结构单元,由于三唑-1-基区域异构体的竞争形成,不易通过标准合成方法以纯形式获得。在研究了多种合成路线后,开发了一种基于通过活化二腙环化构建三唑杂环的方法。在开始 >100 g 规模的演示运行(无需色谱法)之前,必须确定合适的分离方法。新工艺通过四个叠接步骤以 48% 的总收率交付了所需的构建模块,并具有出色的纯度 (99% a/a)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号