Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Insight into the Electronic Effect for Cu Porphyrin Catalysts in Electrocatalytic of CO2 into CH4
Small ( IF 13.0 ) Pub Date : 2023-09-05 , DOI: 10.1002/smll.202304998
Hao Jiang 1 , Peng Zhao 1 , Haidong Shen 1 , Shaowei Yang 1 , Runze Gao 1 , Ying Guo 1, 2 , Yueling Cao 1 , Qiuyu Zhang 1 , Hepeng Zhang 1, 2
Small ( IF 13.0 ) Pub Date : 2023-09-05 , DOI: 10.1002/smll.202304998
Hao Jiang 1 , Peng Zhao 1 , Haidong Shen 1 , Shaowei Yang 1 , Runze Gao 1 , Ying Guo 1, 2 , Yueling Cao 1 , Qiuyu Zhang 1 , Hepeng Zhang 1, 2
Affiliation
|
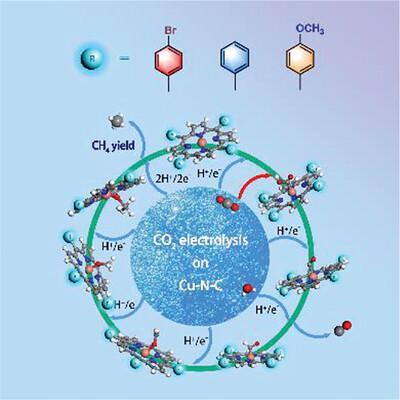
Perturbation of the copper (Cu) active site by electron manipulation is a crucial factor in determining the activity and selectivity of electrochemical carbon dioxide (CO2) reduction reaction (e-CO2RR) in Cu-based molecular catalysts. However, much ambiguity is present concerning their electronic structure–function relationships. Here, three molecular Cu-based porphyrin catalysts with different electron densities at the Cu active site, Cu tetrakis(4-methoxyphenyl)porphyrin (Cu─T(OMe)PP), Cu tetraphenylporphyrin (Cu─THPP), and Cu tetrakis(4-bromophenyl)porphyrin (Cu─TBrPP), are prepared. Although all three catalysts exhibit e-CO2RR activity and the same reaction pathway, their performance is significantly affected by the electronic structure of the Cu site. Theoretical and experimental investigations verify that the conjugated effect of ─OCH3 and ─Br groups lowers the highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbitals (LUMO) gap of Cu─T(OMe)PP and Cu─TBrPP, promoting faster electron transfer between Cu and CO2, thereby improving their e-CO2RR activity. Moreover, the high inductive effect of ─Br group reduces the electron density of Cu active site of Cu─TBrPP, facilitating the hydrolysis of the bound H2O and thus creating a preferable local microenvironment, further enhancing the catalytic performance. This work provides new insights into the relationships between the substituent group characteristics with e-CO2RR performance and is highly instructive for the design of efficient Cu-based e-CO2RR electrocatalysts.
中文翻译:

铜卟啉催化剂电催化 CO2 转化为 CH4 的电子效应的新见解
通过电子操纵对铜(Cu)活性位点的扰动是决定铜基分子催化剂中电化学二氧化碳(CO 2 )还原反应(e-CO 2 RR)活性和选择性的关键因素。然而,它们的电子结构-功能关系还存在很多模糊性。这里,三种分子铜基卟啉催化剂在铜活性位点具有不同的电子密度:四(4-甲氧基苯基)卟啉铜(Cu─T(OMe)PP)、四苯基卟啉铜(Cu─THPP)和四(4)铜制备了-溴苯基)卟啉(Cu─TBrPP)。尽管所有三种催化剂都表现出e-CO 2 RR活性和相同的反应途径,但它们的性能受到Cu位点的电子结构的显着影响。理论和实验研究证实,─OCH 3和─Br基团的共轭效应降低了Cu─T(OMe)PP和Cu─TBrPP的最高占据分子轨道(HOMO)-最低未占据分子轨道(LUMO)间隙,促进了更快的反应Cu和CO 2之间的电子转移,从而提高它们的e-CO 2 RR活性。此外,─Br基团的高诱导效应降低了Cu─TBrPP的Cu活性位点的电子密度,有利于结合的H 2 O的水解,从而创造了良好的局部微环境,进一步增强了催化性能。这项工作为取代基特征与e-CO 2 RR性能之间的关系提供了新的见解,对于设计高效的Cu基e-CO 2 RR电催化剂具有很强的指导意义。
更新日期:2023-09-05
中文翻译:

铜卟啉催化剂电催化 CO2 转化为 CH4 的电子效应的新见解
通过电子操纵对铜(Cu)活性位点的扰动是决定铜基分子催化剂中电化学二氧化碳(CO 2 )还原反应(e-CO 2 RR)活性和选择性的关键因素。然而,它们的电子结构-功能关系还存在很多模糊性。这里,三种分子铜基卟啉催化剂在铜活性位点具有不同的电子密度:四(4-甲氧基苯基)卟啉铜(Cu─T(OMe)PP)、四苯基卟啉铜(Cu─THPP)和四(4)铜制备了-溴苯基)卟啉(Cu─TBrPP)。尽管所有三种催化剂都表现出e-CO 2 RR活性和相同的反应途径,但它们的性能受到Cu位点的电子结构的显着影响。理论和实验研究证实,─OCH 3和─Br基团的共轭效应降低了Cu─T(OMe)PP和Cu─TBrPP的最高占据分子轨道(HOMO)-最低未占据分子轨道(LUMO)间隙,促进了更快的反应Cu和CO 2之间的电子转移,从而提高它们的e-CO 2 RR活性。此外,─Br基团的高诱导效应降低了Cu─TBrPP的Cu活性位点的电子密度,有利于结合的H 2 O的水解,从而创造了良好的局部微环境,进一步增强了催化性能。这项工作为取代基特征与e-CO 2 RR性能之间的关系提供了新的见解,对于设计高效的Cu基e-CO 2 RR电催化剂具有很强的指导意义。

































 京公网安备 11010802027423号
京公网安备 11010802027423号