当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PLP-Dependent Enzyme Methionine γ-Lyase: Insights into the Michaelis Complex from Molecular Dynamics and Free Energy Simulations
Biochemistry ( IF 2.9 ) Pub Date : 2023-09-05 , DOI: 10.1021/acs.biochem.3c00355 Xingyu Chen 1 , Nathan Ferchaud 2 , Pierre Briozzo 2 , David Machover 3 , Thomas Simonson 1
Biochemistry ( IF 2.9 ) Pub Date : 2023-09-05 , DOI: 10.1021/acs.biochem.3c00355 Xingyu Chen 1 , Nathan Ferchaud 2 , Pierre Briozzo 2 , David Machover 3 , Thomas Simonson 1
Affiliation
|
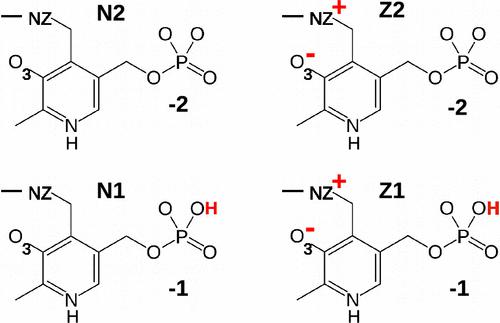
Methionine γ-lyase (MGL) breaks down methionine, with the help of its cofactor pyridoxal-5′-phosphate (PLP), or vitamin B6. Methionine depletion is damaging for cancer cells but not normal cells, so MGL is of interest as a therapeutic protein. To increase our understanding and help engineer improved activity, we focused on the reactive, Michaelis complex between MGL, covalently bound PLP, and substrate Met. is not amenable to crystallography, as it proceeds to products. Experimental activity measurements helped exclude a mechanism that would bypass . We then used molecular dynamics and alchemical free energy simulations to elucidate its structure and dynamics. We showed that the PLP phosphate has a pKa strongly downshifted by the protein, whether Met is present or not. Met binding affects the structure surrounding the reactive atoms. With Met, the Schiff base linkage between PLP and a nearby lysine shifts from a zwitterionic, keto form to a neutral, enol form that makes it easier for Met to approach its labile, target atom. The Met ligand also stabilizes the correct orientation of the Schiff base, more strongly than in simulations without Met, and in agreement with structures in the Protein Data Bank, where the Schiff base orientation correlates with the presence or absence of a co-bound anion or substrate analogue in the active site. Overall, the Met ligand helps organize the active site for the enzyme reaction by reducing fluctuations and shifting protonation states and conformational populations.
中文翻译:

PLP 依赖性酶蛋氨酸 γ-裂解酶:从分子动力学和自由能模拟深入了解米氏复合体
蛋氨酸 γ-裂解酶 (MGL) 在其辅因子吡哆醛-5′-磷酸 (PLP) 或维生素 B6 的帮助下分解蛋氨酸。蛋氨酸消耗会损害癌细胞,但不会损害正常细胞,因此 MGL 作为一种治疗蛋白很受关注。为了增加我们的理解并帮助设计改进的活动,我们专注于反应性米氏复合体MGL、共价结合的 PLP 和底物 Met 之间的关系。不适合晶体学,因为它会产生产物。实验活动测量有助于排除绕过的机制。然后,我们使用分子动力学和炼金术自由能模拟来阐明其结构和动力学。我们发现,无论 Met 是否存在,PLP 磷酸盐的 ap K a都会被蛋白质强烈下调。Met 结合影响反应原子周围的结构。对于 Met,PLP 和附近的赖氨酸之间的希夫碱键从两性离子酮形式转变为中性烯醇形式,这使得 Met 更容易接近其不稳定的目标原子。Met 配体还可以稳定席夫碱的正确方向,比没有 Met 的模拟更稳定,并且与蛋白质数据库中的结构一致,其中席夫碱方向与共结合阴离子或阴离子的存在或不存在相关。活性位点的底物类似物。总体而言,Met 配体通过减少波动和改变质子化状态和构象群来帮助组织酶反应的活性位点。
更新日期:2023-09-05
中文翻译:

PLP 依赖性酶蛋氨酸 γ-裂解酶:从分子动力学和自由能模拟深入了解米氏复合体
蛋氨酸 γ-裂解酶 (MGL) 在其辅因子吡哆醛-5′-磷酸 (PLP) 或维生素 B6 的帮助下分解蛋氨酸。蛋氨酸消耗会损害癌细胞,但不会损害正常细胞,因此 MGL 作为一种治疗蛋白很受关注。为了增加我们的理解并帮助设计改进的活动,我们专注于反应性米氏复合体MGL、共价结合的 PLP 和底物 Met 之间的关系。不适合晶体学,因为它会产生产物。实验活动测量有助于排除绕过的机制。然后,我们使用分子动力学和炼金术自由能模拟来阐明其结构和动力学。我们发现,无论 Met 是否存在,PLP 磷酸盐的 ap K a都会被蛋白质强烈下调。Met 结合影响反应原子周围的结构。对于 Met,PLP 和附近的赖氨酸之间的希夫碱键从两性离子酮形式转变为中性烯醇形式,这使得 Met 更容易接近其不稳定的目标原子。Met 配体还可以稳定席夫碱的正确方向,比没有 Met 的模拟更稳定,并且与蛋白质数据库中的结构一致,其中席夫碱方向与共结合阴离子或阴离子的存在或不存在相关。活性位点的底物类似物。总体而言,Met 配体通过减少波动和改变质子化状态和构象群来帮助组织酶反应的活性位点。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号