当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single atom catalysts for triiodide adsorption and fast conversion to boost the performance of aqueous zinc–iodine batteries
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2023-09-05 , DOI: 10.1039/d3ee01453c Fuhua Yang 1, 2 , Jun Long 3 , Jodie A. Yuwono 1 , Huifang Fei 2 , Yameng Fan 4 , Peng Li 5 , Jinshuo Zou 1 , Junnan Hao 1 , Sailin Liu 1 , Gemeng Liang 1 , Yanqiu Lyu 1 , Xiaobo Zheng 6 , Shiyong Zhao 1 , Kenneth Davey 1 , Zaiping Guo 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2023-09-05 , DOI: 10.1039/d3ee01453c Fuhua Yang 1, 2 , Jun Long 3 , Jodie A. Yuwono 1 , Huifang Fei 2 , Yameng Fan 4 , Peng Li 5 , Jinshuo Zou 1 , Junnan Hao 1 , Sailin Liu 1 , Gemeng Liang 1 , Yanqiu Lyu 1 , Xiaobo Zheng 6 , Shiyong Zhao 1 , Kenneth Davey 1 , Zaiping Guo 1
Affiliation
|
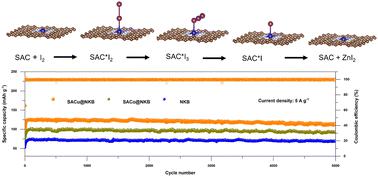
Zinc–iodine (Zn–I2) batteries are promising for energy storage because of their low cost, environmental friendliness, and attractive energy density. However, triiodide dissolution and poor conversion kinetics hinder their application. Herein, we demonstrated that the ‘shuttle effect’ in Zn–I2 batteries can be suppressed via single atom catalyst (SAC) cathodes because of efficient catalytic activity in I2/I3−/I− reactions and their ability to adsorb I3−. Based on DFT computations, an I− poisoning mechanism was proposed for SAC selection to suppress the shuttle effect in Zn–I2 batteries. I− formation and desorption are crucial to maintaining the catalytic and adsorption role of metallic elements. SACu favours the reduction of I2 to I and exhibits a low energy barrier to release I− from the surface, thus allowing more rapid conversion kinetics, while at the same time suppressing the shuttle effect of I3− in Zn–I2 batteries. In contrast, without sufficient energy, the final product of I− will remain adsorbed at the metal site of SAFe, SAMn, SAV, and SATi, thus killing the catalytic activity of SACs to facilitate the iodine reduction reaction (IRR). To confirm practicality, single-atom Cu-embedded nitrogen-doped Ketjen black (SACu@NKB), together with SACo@NKB and NKB, were synthesized and electrochemically assessed. The as-prepared SACu@NKB outperformed the SACo@NKB and NKB cathodes in terms of reversible capacity and cycle life. In addition, a rate-limiting step in these redox reactions was identified, and overpotential was estimated, and these were found to be dependent on the d-band centre of SACs. A lower d-band centre can be associated with more optimal catalytic performance in SACs. This work reveals that the superior cycle life of Zn–I2 batteries is underpinned by the catalytic and adsorption role of metallic catalysts, and we report an in-depth understanding of how this boosts the performance of Zn–I2 batteries, with implications for future long-life battery design.
中文翻译:

用于三碘化物吸附和快速转化的单原子催化剂可提高水性锌碘电池的性能
锌-碘(Zn-I 2)电池因其成本低、环境友好和有吸引力的能量密度而在储能方面很有前景。然而,三碘化物溶解和较差的转化动力学阻碍了它们的应用。在此,我们证明,由于 I 2 /I 3 - /I -反应中的高效催化活性及其吸附 I 3 的能力,可以通过单原子催化剂(SAC)阴极抑制Zn-I 2电池中的“穿梭效应” - . 基于DFT计算,提出了一种I -中毒机制用于SAC选择,以抑制Zn-I 2中的穿梭效应电池。I - 的形成和解吸对于维持金属元素的催化和吸附作用至关重要。SACu有利于I 2还原为I,并表现出低能垒以从表面释放I -,从而允许更快速的转换动力学,同时抑制Zn-I 2电池中I 3 -的穿梭效应。相反,如果没有足够的能量,I 的最终产物-将继续吸附在 SAFe、SAMn、SAV 和 SATi 的金属位点上,从而杀死 SAC 的催化活性,从而促进碘还原反应(IRR)。为了确认实用性,合成了单原子嵌入铜的氮掺杂科琴黑 (SACu@NKB) 以及 SACo@NKB 和 NKB,并进行了电化学评估。所制备的 SACu@NKB 在可逆容量和循环寿命方面优于 SACo@NKB 和 NKB 正极。此外,还确定了这些氧化还原反应中的限速步骤,并估计了过电势,并且发现这些步骤取决于 SAC 的 d 带中心。较低的 d 带中心可能与 SAC 中更优化的催化性能相关。这项工作揭示了 Zn-I 2优异的循环寿命电池的基础是金属催化剂的催化和吸附作用,我们报告了对这如何提高 Zn-I 2电池性能的深入了解,对未来长寿命电池设计的影响。
更新日期:2023-09-05
中文翻译:

用于三碘化物吸附和快速转化的单原子催化剂可提高水性锌碘电池的性能
锌-碘(Zn-I 2)电池因其成本低、环境友好和有吸引力的能量密度而在储能方面很有前景。然而,三碘化物溶解和较差的转化动力学阻碍了它们的应用。在此,我们证明,由于 I 2 /I 3 - /I -反应中的高效催化活性及其吸附 I 3 的能力,可以通过单原子催化剂(SAC)阴极抑制Zn-I 2电池中的“穿梭效应” - . 基于DFT计算,提出了一种I -中毒机制用于SAC选择,以抑制Zn-I 2中的穿梭效应电池。I - 的形成和解吸对于维持金属元素的催化和吸附作用至关重要。SACu有利于I 2还原为I,并表现出低能垒以从表面释放I -,从而允许更快速的转换动力学,同时抑制Zn-I 2电池中I 3 -的穿梭效应。相反,如果没有足够的能量,I 的最终产物-将继续吸附在 SAFe、SAMn、SAV 和 SATi 的金属位点上,从而杀死 SAC 的催化活性,从而促进碘还原反应(IRR)。为了确认实用性,合成了单原子嵌入铜的氮掺杂科琴黑 (SACu@NKB) 以及 SACo@NKB 和 NKB,并进行了电化学评估。所制备的 SACu@NKB 在可逆容量和循环寿命方面优于 SACo@NKB 和 NKB 正极。此外,还确定了这些氧化还原反应中的限速步骤,并估计了过电势,并且发现这些步骤取决于 SAC 的 d 带中心。较低的 d 带中心可能与 SAC 中更优化的催化性能相关。这项工作揭示了 Zn-I 2优异的循环寿命电池的基础是金属催化剂的催化和吸附作用,我们报告了对这如何提高 Zn-I 2电池性能的深入了解,对未来长寿命电池设计的影响。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号