当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of an Intramolecular Aryne Ene Reaction and Application to the Formal Synthesis of (±)-Crinine
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-09-06 , DOI: 10.1021/ja306881u
David A. Candito 1 , Dennis Dobrovolsky 1 , Mark Lautens 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-09-06 , DOI: 10.1021/ja306881u
David A. Candito 1 , Dennis Dobrovolsky 1 , Mark Lautens 1
Affiliation
|
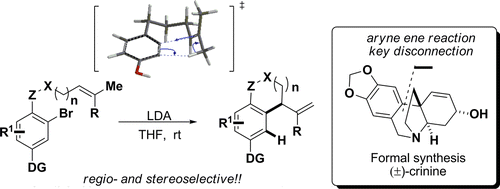
A general and high yielding annulation strategy for the synthesis of various carbo- and heterocycles, based on an intramolecular aryne ene reaction is described. It was found that the geometry of the olefin is crucial to the success of the reaction, with exclusive migration of the trans-allylic-H taking place. Furthermore, the electronic nature of the aryne was found to be important to the success of the reaction. Deuterium labeling studies and DFT calculations provided insight into the reaction mechanism. The data suggests a concerted asynchronous transition state, resembling a nucleophilic attack on the aryne. This strategy was successfully applied to the formal synthesis of the ethanophenanthridine alkaloid (±)-crinine.
中文翻译:

分子内芳炔反应的发展及其在 (±)-Crinine 的正式合成中的应用
描述了基于分子内亚芳烯反应合成各种碳环和杂环的通用和高产环化策略。发现烯烃的几何形状对反应的成功至关重要,因为发生反式烯丙基-H 的专属迁移。此外,发现芳炔的电子性质对反应的成功很重要。氘标记研究和 DFT 计算提供了对反应机理的深入了解。数据表明一个协调的异步过渡态,类似于对芳炔的亲核攻击。该策略已成功应用于乙酰菲啶生物碱 (±)-crinine 的正式合成。
更新日期:2012-09-06
中文翻译:

分子内芳炔反应的发展及其在 (±)-Crinine 的正式合成中的应用
描述了基于分子内亚芳烯反应合成各种碳环和杂环的通用和高产环化策略。发现烯烃的几何形状对反应的成功至关重要,因为发生反式烯丙基-H 的专属迁移。此外,发现芳炔的电子性质对反应的成功很重要。氘标记研究和 DFT 计算提供了对反应机理的深入了解。数据表明一个协调的异步过渡态,类似于对芳炔的亲核攻击。该策略已成功应用于乙酰菲啶生物碱 (±)-crinine 的正式合成。




































 京公网安备 11010802027423号
京公网安备 11010802027423号