European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-09-04 , DOI: 10.1016/j.ejmech.2023.115790 Martin Loffelmann 1 , Zdeněk Škrott 1 , Dušana Majera 1 , Pavel Štarha 2 , Vladimír Kryštof 3 , Martin Mistrík 1
|
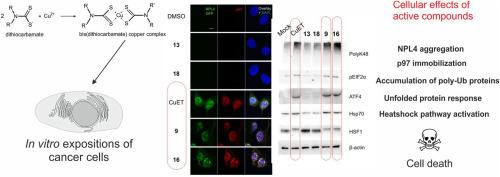
Dithiocarbamates (DTCs) are simple organic compounds with many applications in industry and medicine. They are potent metal chelators forming complexes with various metal ions, including copper. Recently, bis(diethyldithiocarbamate)-copper complex (CuET) has been identified as a metabolic product of the anti-alcoholic drug Antabuse (disulfiram, DSF), standing behind DSF's reported anticancer activity. Mechanistically, CuET in cells causes aggregation of NPL4 protein, an essential cofactor of the p97 segregase, an integral part of the ubiquitin-proteasome system. The malfunction of p97/NPL4 caused by CuET leads to proteotoxic stress accompanied by heat shock and unfolded protein responses and cancer cell death. However, it is not known whether the NPL4 inhibition is unique for CuET or whether it is shared with other dithiocarbamate-copper complexes. Thus, we tested 20 DTCs-copper complexes in this work for their ability to target and aggregate NPL4 protein. Surprisingly, we have found that certain potency against NPL4 is relatively common for structurally different DTCs-copper complexes, as thirteen compounds scored in the cellular NPL4 aggregation assay. These compounds also shared typical cellular phenotypes reported previously for CuET, including the NPL4/p97 proteins immobilization, accumulation of polyubiquitinated proteins, the unfolded protein, and the heat shock responses. Moreover, the active complexes were also toxic to cancer cells (the most potent in the nanomolar range), and we have found a strong positive correlation between NPL4 aggregation and cytotoxicity, confirming NPL4 as a relevant target. These results show the widespread potency of DTCs-copper complexes to target NPL4 with subsequent induction of lethal proteotoxic stress in cancer cells with implications for drug development.
中文翻译:

鉴定癌细胞中靶向 p97/NPL4 通路的新型二硫代氨基甲酸酯-铜复合物
二硫代氨基甲酸酯 (DTC) 是简单的有机化合物,在工业和医学中有许多应用。它们是强效金属螯合剂,可与各种金属离子(包括铜)形成络合物。最近,双(二乙基二硫代氨基甲酸酯)-铜复合物 (CuET) 已被确定为抗酒精药物 Antabuse(双硫仑,DSF)的代谢产物,支持 DSF 报道的抗癌活性。从机制上讲,细胞中的 CuET 导致 NPL4 蛋白聚集,NPL4 蛋白是 p97 分离酶的重要辅因子,是泛素-蛋白酶体系统的组成部分。CuET 引起的 p97/NPL4 功能障碍导致蛋白毒性应激伴有热休克和未折叠的蛋白质反应和癌细胞死亡。然而,尚不清楚 NPL4 抑制是否是 CuET 独有的,或者它是否与其他二硫代氨基甲酸酯-铜复合物共享。因此,我们在这项工作中测试了 20 个 DTCs-铜复合物靶向和聚集 NPL4 蛋白的能力。令人惊讶的是,我们发现对于结构不同的 DTCs-铜复合物,对 NPL4 的某些效力相对常见,因为在细胞 NPL4 聚集测定中对 13 种化合物进行了评分。这些化合物还共享先前报道的 CuET 的典型细胞表型,包括 NPL4/p97 蛋白固定、多泛素化蛋白的积累、未折叠的蛋白和热休克反应。此外,活性复合物对癌细胞也有毒性(在纳摩尔范围内最有效),我们发现 NPL4 聚集与细胞毒性之间存在很强的正相关关系,证实 NPL4 是一个相关靶标。 这些结果表明,DTCs-铜复合物靶向 NPL4 的广泛效力,随后在癌细胞中诱导致命的蛋白毒性应激,对药物开发有影响。















































 京公网安备 11010802027423号
京公网安备 11010802027423号