当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical C–H Arylation of Quinazolinone with Aryl Tetrafluoroborate Diazonium Salts in Aqueous Solution of Ionic Liquids
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-09-01 , DOI: 10.1021/acssuschemeng.3c04247 Mengmeng Ji 1 , Weiya Zhang 1 , Hongbo Zhao 1 , Yuying Gu 1 , Wenyi Chu 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-09-01 , DOI: 10.1021/acssuschemeng.3c04247 Mengmeng Ji 1 , Weiya Zhang 1 , Hongbo Zhao 1 , Yuying Gu 1 , Wenyi Chu 1
Affiliation
|
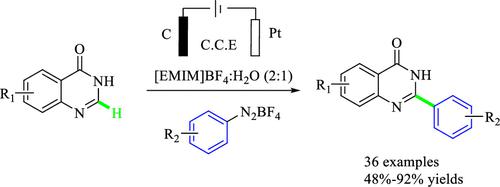
A process for the electrochemical direct C–H arylation of quinazolinones and aryl tetrafluoroborate diazonium salts with ionic liquid [EMIM]BF4 (1-ethyl-3-methylimidazolium tetrafluoroborate):H2O (2:1) as electrolyte was developed for the synthesis of 2-arylquinazolinones, and a series of 2-arylquinazolinones were synthesized with moderate to good yields. This method was applied for the synthesis of a key intermediate of an IKK β inhibitor CU160 and a quinazolinone alkaloid 2-(1H-indol-3-yl) quinazolin-4(3H)-one. The mild and green synthesis scheme provides an effective alternative to the traditional synthesis of 2-arylquinazolinone.
中文翻译:

离子液体水溶液中喹唑啉酮与芳基四氟硼酸重氮盐的电化学 C-H 芳基化
开发了一种以离子液体[EMIM]BF 4 (1-乙基-3-甲基咪唑鎓四氟硼酸盐):H 2 O (2:1)为电解质对喹唑啉酮和芳基四氟硼酸重氮盐进行电化学直接C-H芳基化的方法。2-芳基喹唑啉酮的合成,并合成了一系列2-芳基喹唑啉酮,产率中等至良好。该方法用于合成IKKβ抑制剂CU160和喹唑啉酮生物碱2-(1H-吲哚-3-基)喹唑啉-4(3H ) -酮的关键中间体。该温和、绿色的合成方案为传统2-芳基喹唑啉酮的合成提供了有效的替代方案。
更新日期:2023-09-01
中文翻译:

离子液体水溶液中喹唑啉酮与芳基四氟硼酸重氮盐的电化学 C-H 芳基化
开发了一种以离子液体[EMIM]BF 4 (1-乙基-3-甲基咪唑鎓四氟硼酸盐):H 2 O (2:1)为电解质对喹唑啉酮和芳基四氟硼酸重氮盐进行电化学直接C-H芳基化的方法。2-芳基喹唑啉酮的合成,并合成了一系列2-芳基喹唑啉酮,产率中等至良好。该方法用于合成IKKβ抑制剂CU160和喹唑啉酮生物碱2-(1H-吲哚-3-基)喹唑啉-4(3H ) -酮的关键中间体。该温和、绿色的合成方案为传统2-芳基喹唑啉酮的合成提供了有效的替代方案。































 京公网安备 11010802027423号
京公网安备 11010802027423号