当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NH3 Decomposition for H2 Generation: Effects of Cheap Metals and Supports on Plasma–Catalyst Synergy
ACS Catalysis ( IF 11.3 ) Pub Date : 2015-06-11 00:00:00 , DOI: 10.1021/acscatal.5b00728 Li Wang 1 , Yanhui Yi 1 , Yue Zhao 1 , Rui Zhang 1 , Jialiang Zhang 2 , Hongchen Guo 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2015-06-11 00:00:00 , DOI: 10.1021/acscatal.5b00728 Li Wang 1 , Yanhui Yi 1 , Yue Zhao 1 , Rui Zhang 1 , Jialiang Zhang 2 , Hongchen Guo 1
Affiliation
|
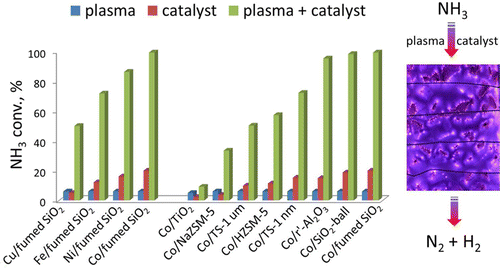
NH3 decomposition is important because of its potential use in generating CO-free H2. In this study, several cheap metals (Fe, Co, Ni, and Cu) and a series of supports (zeolite materials: TS-1 um, TS-1 nm, HZSM-5 nm, and NaZSM-5 nm; SiO2-based materials: fumed SiO2 and SiO2-ball; and metal oxide materials: r′-Al2O3 and TiO2) were used to prepare supported catalysts. X-ray fluorescence, N2 physisorption, X-ray diffraction, transmission electron microscopy, Fourier-transform infrared spectroscopy, temperature-programmed desorption, mass spectrometry, temperature-programmed reduction, and electrical property analysis were used to investigate the effect of the catalyst on the synergy between a plasma (produced by dielectric barrier discharge) and the catalyst in NH3 decomposition. The results show that the synergy depends strongly on the strength of the metal–nitrogen (M–N) bond, and the relative dielectric constant (εd) of the support. When Fe, Co, Ni, and Cu were supported on fumed SiO2, the order of the strengths of the M–N bonds was Cu–N < Ni–N < Co–N < Fe–N. Among the catalysts, Co/fumed SiO2 showed a stronger synergy with the plasma and gave higher NH3 conversion in plasma catalysis. Co catalysts supported on fumed SiO2, SiO2-ball, and r′-Al2O3, which have small εd values, had stronger synergies with plasma and therefore gave higher NH3 conversions. The relative dielectric constant of the support correlated well with NH3 conversion in plasma catalysis. These results show that the relative dielectric constant is an essential parameter in developing catalyst supports for plasma conditions. This study provides direct proof that the recombinative desorption of adsorbed N atoms is the rate-limiting step in the catalytic decomposition of NH3 over cheap metal catalysts such as Fe, Co, and Ni and that there is synergy between plasma and cheap metal catalysts in plasma-catalytic NH3 decomposition.
中文翻译:

H 2生成的NH 3分解:廉价金属和载体对等离子体-催化剂协同作用的影响
NH 3分解很重要,因为它潜在地用于生成不含CO的H 2。在这项研究中,一些便宜的金属(铁,钴,镍,和铜)和一系列支撑件(沸石材料:TS-1微米,TS-1纳米,HZSM-5纳米,且NaZSM-5纳米;的SiO 2 -基材料:热解法SiO 2和SiO 2-球;金属氧化物材料:r'-Al 2 O 3和TiO 2)用于制备负载型催化剂。X射线荧光,N 2物理吸附,X射线衍射,透射电子显微镜,傅立叶变换红外光谱,程序升温脱附,质谱,程序升温还原和电性能分析被用于研究催化剂对等离子体之间协同作用的影响(介电势垒放电产生的产物)和催化剂在NH 3中的分解。该结果表明,该协同作用强烈地依赖于金属-氮的强度(M-N)键,相对介电常数(ε d载体的)。当将Fe,Co,Ni和Cu负载在热解SiO 2上时,M–N键的强度顺序为Cu–N <Ni–N <Co–N <Fe–N。在催化剂中,Co /热解SiO 2在等离子体催化中显示出与等离子体更强的协同作用,并提供更高的NH 3转化率。支撑在热解法二氧化硅Co催化剂2的SiO 2 -ball,和R'-的Al 2 ö 3,其具有小的ε d值,与血浆更强的协同作用,因此,得到更高的NH 3转化率。载体的相对介电常数与NH 3相关性很好在等离子体催化中的转化。这些结果表明,相对介电常数是开发用于等离子体条件的催化剂载体的基本参数。这项研究提供了直接的证据,证明吸附的N原子的重组解吸是在廉价的金属催化剂(如Fe,Co和Ni)上催化NH 3催化分解的速率限制步骤,并且等离子体和廉价的金属催化剂之间存在协同作用。等离子体催化NH 3分解。
更新日期:2015-06-11
中文翻译:

H 2生成的NH 3分解:廉价金属和载体对等离子体-催化剂协同作用的影响
NH 3分解很重要,因为它潜在地用于生成不含CO的H 2。在这项研究中,一些便宜的金属(铁,钴,镍,和铜)和一系列支撑件(沸石材料:TS-1微米,TS-1纳米,HZSM-5纳米,且NaZSM-5纳米;的SiO 2 -基材料:热解法SiO 2和SiO 2-球;金属氧化物材料:r'-Al 2 O 3和TiO 2)用于制备负载型催化剂。X射线荧光,N 2物理吸附,X射线衍射,透射电子显微镜,傅立叶变换红外光谱,程序升温脱附,质谱,程序升温还原和电性能分析被用于研究催化剂对等离子体之间协同作用的影响(介电势垒放电产生的产物)和催化剂在NH 3中的分解。该结果表明,该协同作用强烈地依赖于金属-氮的强度(M-N)键,相对介电常数(ε d载体的)。当将Fe,Co,Ni和Cu负载在热解SiO 2上时,M–N键的强度顺序为Cu–N <Ni–N <Co–N <Fe–N。在催化剂中,Co /热解SiO 2在等离子体催化中显示出与等离子体更强的协同作用,并提供更高的NH 3转化率。支撑在热解法二氧化硅Co催化剂2的SiO 2 -ball,和R'-的Al 2 ö 3,其具有小的ε d值,与血浆更强的协同作用,因此,得到更高的NH 3转化率。载体的相对介电常数与NH 3相关性很好在等离子体催化中的转化。这些结果表明,相对介电常数是开发用于等离子体条件的催化剂载体的基本参数。这项研究提供了直接的证据,证明吸附的N原子的重组解吸是在廉价的金属催化剂(如Fe,Co和Ni)上催化NH 3催化分解的速率限制步骤,并且等离子体和廉价的金属催化剂之间存在协同作用。等离子体催化NH 3分解。















































 京公网安备 11010802027423号
京公网安备 11010802027423号