Separation and Purification Technology ( IF 8.1 ) Pub Date : 2023-09-01 , DOI: 10.1016/j.seppur.2023.124916 Yalan Zhang , Xun Jia , Guizhou Xu , Wei Liu , Du Hu , Qianqian Sun , Jinying Xu , Guihai Zhang , Wenrong Xiong , Zhifei Ma , Yongdong Zhang , Jianjun Dai , Huike zhou , Daishe Wu , Xianchuan Xie
|
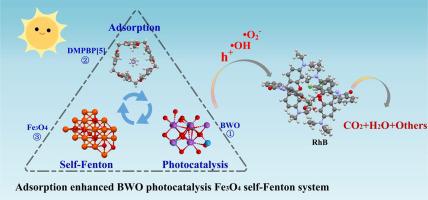
In this study, a DMP5 polymer (DMPBP[5]) adsorption enhanced Bi2WO6 (BWO) photocatalysis Fe3O4 self-Fenton system (DMPBP[5]-BWO-Fe3O4) was proposed. The system includes the adsorption of pollutants and active substances by DMPBP[5] as the prerequisite and driving force for the reaction, a Bi-based photocatalyst as an in situ robust H2O2 generator, and decorated Fe3O4 (Fe2+/Fe3+) as the trigger and enhancer for H2O2 formation and transformation, which leads to a highly efficient and stable activity for persistent organic pollutants. Considering the photo-oxidation of RhB as a model reaction, this system exhibits 17–18 times higher photodegradation rate than that of the original BWO, which corresponds to a high catalytic activity of 98.3% (k = 14.2 × 10-3 min−1) within 180 min. The advantages of the system are mainly attributed to: (1) DMPBP[5] has a large specific surface area (1386 m2/g), which provides more adsorption sites for pollutants; (2) Further, through theoretical calculation, we found that the super-strong adsorption capacity of DMPBP[5] for RhB (−0.061 eV) was much higher than that of Fe3O4 (−0.0025 eV) and BWO (−0.0015 eV); (3) The production of H2O2 on DMPBP[5]-BWO-Fe3O4 is greatly increased, and the concentration of H2O2 can be accumulated to 5.506 μM within 120 min, which is about 90.3 times and 9.25 times higher than that of BWO and BWO-D7, respectively. The system presented here provides new insights into the efficient and environmentally friendly removal of refractory organic contaminants.
中文翻译:

DMPBP[5]吸附增强Bi2WO6光催化Fe3O4自Fenton体系的理论研究与实验验证
本研究提出了一种DMP5聚合物(DMPBP[ 5 ])吸附增强Bi 2 WO 6 (BWO)光催化Fe 3 O 4自芬顿体系(DMPBP[ 5 ]-BWO-Fe 3 O 4 )。该系统包括以DMPBP[ 5 ]吸附污染物和活性物质作为反应的前提和驱动力,Bi基光催化剂作为原位稳健的H 2 O 2发生器,以及修饰的Fe 3 O 4 (Fe 2 + /Fe 3+ ) 作为 H 2 O 2的触发剂和增强剂形成和转化,从而实现对持久性有机污染物的高效稳定的活性。以RhB的光氧化为模型反应,该系统的光降解率比原始BWO高17-18倍,相当于98.3%的高催化活性(k = 14.2 × 10 -3 min -1)180分钟内。该系统的优点主要归因于:(1)DMPBP[ 5 ]具有较大的比表面积(1386 m 2 /g),为污染物提供了更多的吸附位点;(2)进一步,通过理论计算,我们发现DMPBP[5]对RhB(-0.061 eV)的超强吸附能力远高于Fe 3 O 4(-0.0025 eV) 和 BWO (-0.0015 eV);(3) DMPBP[5]-BWO-Fe 3 O 4上H 2 O 2产量大幅增加,120 min内H 2 O 2浓度可累积至5.506 μM,约为原来的90.3倍。分别比BWO和BWO-D 7高9.25倍。这里介绍的系统为高效、环保地去除难降解有机污染物提供了新的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号