Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enlarging the Ni–O Bond Polarizability in a Phosphorene-Hosted Metal–Organic Framework for Boosted Water Oxidation Electrocatalysis
ACS Nano ( IF 15.8 ) Pub Date : 2023-08-31 , DOI: 10.1021/acsnano.3c05224
Wenfang Zhai 1 , Ya Chen 1 , Yaoda Liu 1 , Thangavel Sakthivel 1 , Yuanyuan Ma 2 , Yuanbin Qin 1 , Yongquan Qu 2 , Zhengfei Dai 1
ACS Nano ( IF 15.8 ) Pub Date : 2023-08-31 , DOI: 10.1021/acsnano.3c05224
Wenfang Zhai 1 , Ya Chen 1 , Yaoda Liu 1 , Thangavel Sakthivel 1 , Yuanyuan Ma 2 , Yuanbin Qin 1 , Yongquan Qu 2 , Zhengfei Dai 1
Affiliation
|
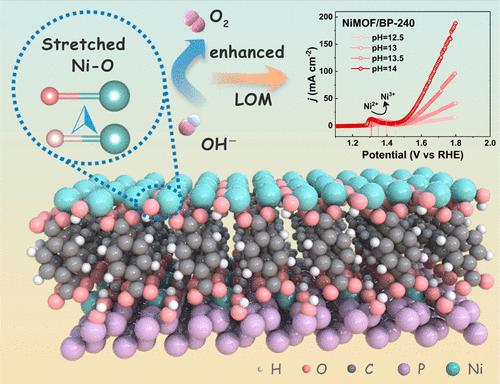
The emerging lattice-oxygen oxidation mechanism (LOM) presents attractive opportunities for breaking the scaling relationship to boost oxygen evolution reaction (OER) with the direct OLattice–*O interaction. However, currently the LOM-triggering rationales are still debated, and a streamlined physicochemical paradigm is extremely desirable for the design of LOM-defined OER catalysts. Herein, a Ni metal–organic framework/black phosphorene (NiMOF/BP) heterostructure is theoretically profiled and constructed as a catalytic platform for the LOM-derived OER studies. It is found that the p-type BP host can enlarge the Ni–O bond polarizability of NiMOF through the Ni–O bond stretching and Ni valence declining synergically. Such an enlarged bond polarizability will in principle alleviate the lattice oxygen confinement to benefit the LOM pathway and OER performance. As a result, the optimized NiMOF/BP catalyst exhibits promising OER performance with a low overpotential of 260 mV at 10 mA cm–2 and long-term stability in 1 M KOH electrolyte. Both experiment and calculation results suggest the activated LOM pathway with a more balanced step barrier in the NiMOF/BP OER catalyst. This research puts forward Ni–O bond polarizability as the criterion to design LOM-scaled electrocatalysts for water oxidation.
中文翻译:

增大磷烯金属有机框架中 Ni-O 键的极化率,用于增强水氧化电催化
新兴的晶格-氧氧化机制(LOM)为打破比例关系提供了诱人的机会,通过直接的O晶格-*O相互作用来促进析氧反应(OER) 。然而,目前 LOM 触发原理仍然存在争议,并且对于 LOM 定义的 OER 催化剂的设计来说,简化的物理化学范式是非常理想的。在此,从理论上描述并构建了镍金属有机骨架/黑磷烯(NiMOF/BP)异质结构,并将其构建为LOM衍生的OER研究的催化平台。研究发现,p型BP主体可以通过Ni-O键拉伸和Ni价态下降协同增大NiMOF的Ni-O键极化率。这种增大的键极化率原则上将减轻晶格氧限制,从而有利于 LOM 途径和 OER 性能。因此,优化的 NiMOF/BP 催化剂表现出良好的 OER 性能,在 10 mA cm –2下具有 260 mV 的低过电势,并且在 1 M KOH 电解质中具有长期稳定性。实验和计算结果都表明,NiMOF/BP OER 催化剂中激活的 LOM 途径具有更平衡的阶梯势垒。本研究提出以 Ni-O 键极化率作为设计 LOM 级水氧化电催化剂的标准。
更新日期:2023-08-31
中文翻译:

增大磷烯金属有机框架中 Ni-O 键的极化率,用于增强水氧化电催化
新兴的晶格-氧氧化机制(LOM)为打破比例关系提供了诱人的机会,通过直接的O晶格-*O相互作用来促进析氧反应(OER) 。然而,目前 LOM 触发原理仍然存在争议,并且对于 LOM 定义的 OER 催化剂的设计来说,简化的物理化学范式是非常理想的。在此,从理论上描述并构建了镍金属有机骨架/黑磷烯(NiMOF/BP)异质结构,并将其构建为LOM衍生的OER研究的催化平台。研究发现,p型BP主体可以通过Ni-O键拉伸和Ni价态下降协同增大NiMOF的Ni-O键极化率。这种增大的键极化率原则上将减轻晶格氧限制,从而有利于 LOM 途径和 OER 性能。因此,优化的 NiMOF/BP 催化剂表现出良好的 OER 性能,在 10 mA cm –2下具有 260 mV 的低过电势,并且在 1 M KOH 电解质中具有长期稳定性。实验和计算结果都表明,NiMOF/BP OER 催化剂中激活的 LOM 途径具有更平衡的阶梯势垒。本研究提出以 Ni-O 键极化率作为设计 LOM 级水氧化电催化剂的标准。































 京公网安备 11010802027423号
京公网安备 11010802027423号