Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2023-08-30 , DOI: 10.1016/j.jelechem.2023.117758
Xiaoxin Lv , Yan Zhang , Lin Wen , Aomen Yang , Jun Liang
|
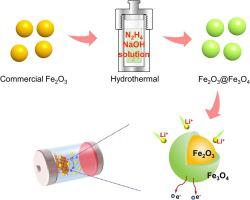
Fe2O3 has been considered as a promising anode material for lithium-ion batteries (LIBs) owing to its high specific capacity. However, its sluggish charge transfer resulting from the poor electrical conductivity severely limits the electrochemical performance. In this work, an ultrathin Fe3O4 layer-coated Fe2O3 composite was fabricated through a facile one-step hydrothermal method with hydrazine hydrate as the reductant in an alkaline environment. Upon use as the anode for LIBS, the as-resulted Fe2O3@Fe3O4 composite achieves a high specific capacity of 1539.5 mA h g−1 at a current density of 100 mA g−1 and simultaneously maintains a high discharge capacity of 707.8 mAh g−1 after 800 cycles at 1000 mA g−1, outperforming the commercial Fe2O3 sample. Electrochemical characterizations reveal that the improved electrochemical performance can be attributed to the combined effects of higher theoretical specific capacity of Fe2O3 and superior electrical conductivity of Fe3O4.
中文翻译:

轻松合成 Fe3O4 超薄层涂层 Fe2O3 复合阳极以增强锂离子存储
Fe 2 O 3由于其高比容量而被认为是一种有前途的锂离子电池(LIB)负极材料。然而,其导电性差导致电荷转移缓慢,严重限制了其电化学性能。本工作以水合肼为还原剂,在碱性环境下,通过简便的一步水热法制备了超薄Fe 3 O 4层包覆的Fe 2 O 3复合材料。当用作LIBS阳极时,所得的Fe 2 O 3 @Fe 3 O 4复合材料实现了1539.5 mAh g -1的高比容量在100 mA g -1的电流密度下,同时在1000 mA g -1下循环800次后仍保持707.8 mAh g -1的高放电容量,优于商业Fe 2 O 3样品。电化学表征表明,电化学性能的提高可归因于Fe 2 O 3较高的理论比容量和Fe 3 O 4优异的电导率的综合作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号