当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantifying the Effect of Guest Binding on Host Environment
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-08-29 , DOI: 10.1021/jacs.3c01409
Hugh P Ryan 1 , Zachary S Fishman 2 , Jacob T Pawlik 2 , Angela Grommet 1 , Malgorzata Musial 3 , Felix Rizzuto 1 , James C Booth 2 , Christian J Long 2 , Kathleen Schwarz 3 , Nathan D Orloff 2 , Jonathan R Nitschke 1 , Angela C Stelson 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-08-29 , DOI: 10.1021/jacs.3c01409
Hugh P Ryan 1 , Zachary S Fishman 2 , Jacob T Pawlik 2 , Angela Grommet 1 , Malgorzata Musial 3 , Felix Rizzuto 1 , James C Booth 2 , Christian J Long 2 , Kathleen Schwarz 3 , Nathan D Orloff 2 , Jonathan R Nitschke 1 , Angela C Stelson 2
Affiliation
|
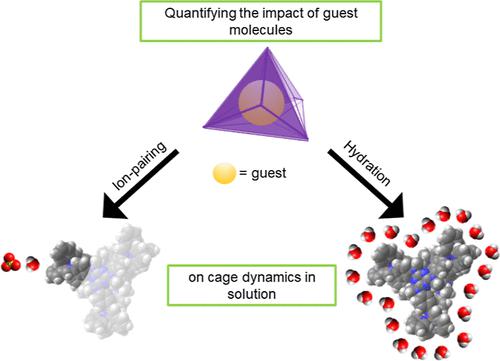
The environment around a host–guest complex is defined by intermolecular interactions between the complex, solvent molecules, and counterions. These interactions govern both the solubility of these complexes and the rates of reactions occurring within the host molecules and can be critical to catalytic and separation applications of host–guest systems. However, these interactions are challenging to detect using standard analytical chemistry techniques. Here, we quantify the hydration and ion pairing of a FeII4L4 coordination cage with a set of guest molecules having widely varying physicochemical properties. The impact of guest properties on host ion pairing and hydration was determined through microwave microfluidic measurements paired with principal component analysis (PCA). This analysis showed that introducing guest molecules into solution displaced counterions that were bound to the cage, and that the solvent solubility of the guest has the greatest impact on the solvent and ion-pairing dynamics surrounding the host. Specifically, we found that when we performed PCA of the measured equivalent circuit parameters and the solubility and dipole moment, we observed a high (>90%) explained variance for the first two principal components for each circuit parameter. We also observed that cage-counterion pairing is well-described by a single ion-pairing type, with a one-step reaction model independent of the type of cargo, and that the ion-pairing association constant is reduced for cargo with higher water solubility. Quantifying hydration and cage-counterion interactions is a critical step to building the next generation of design criteria for host–guest chemistries.
中文翻译:

量化来宾绑定对主机环境的影响
主客体复合物周围的环境由复合物、溶剂分子和反离子之间的分子间相互作用定义。这些相互作用控制着这些复合物的溶解度和主体分子内发生的反应速率,并且对于主客体系统的催化和分离应用至关重要。然而,使用标准分析化学技术检测这些相互作用具有挑战性。在这里,我们量化了 Fe II 4 L 4配位笼与一组具有广泛不同物理化学性质的客体分子的水合和离子配对。通过微波微流体测量结合主成分分析 (PCA) 确定客体特性对主体离子配对和水合的影响。该分析表明,将客体分子引入溶液中会取代与笼结合的抗衡离子,并且客体的溶剂溶解度对主体周围的溶剂和离子对动力学影响最大。具体来说,我们发现,当我们对测量的等效电路参数以及溶解度和偶极矩进行 PCA 时,我们观察到每个电路参数的前两个主成分的解释方差较高 (>90%)。我们还观察到,笼-抗衡离子配对由单一离子对类型很好地描述,具有独立于货物类型的一步反应模型,并且对于具有较高水溶性的货物,离子对缔合常数降低。量化水合和笼-反离子相互作用是建立下一代主客体化学设计标准的关键步骤。
更新日期:2023-08-29
中文翻译:

量化来宾绑定对主机环境的影响
主客体复合物周围的环境由复合物、溶剂分子和反离子之间的分子间相互作用定义。这些相互作用控制着这些复合物的溶解度和主体分子内发生的反应速率,并且对于主客体系统的催化和分离应用至关重要。然而,使用标准分析化学技术检测这些相互作用具有挑战性。在这里,我们量化了 Fe II 4 L 4配位笼与一组具有广泛不同物理化学性质的客体分子的水合和离子配对。通过微波微流体测量结合主成分分析 (PCA) 确定客体特性对主体离子配对和水合的影响。该分析表明,将客体分子引入溶液中会取代与笼结合的抗衡离子,并且客体的溶剂溶解度对主体周围的溶剂和离子对动力学影响最大。具体来说,我们发现,当我们对测量的等效电路参数以及溶解度和偶极矩进行 PCA 时,我们观察到每个电路参数的前两个主成分的解释方差较高 (>90%)。我们还观察到,笼-抗衡离子配对由单一离子对类型很好地描述,具有独立于货物类型的一步反应模型,并且对于具有较高水溶性的货物,离子对缔合常数降低。量化水合和笼-反离子相互作用是建立下一代主客体化学设计标准的关键步骤。

































 京公网安备 11010802027423号
京公网安备 11010802027423号