Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-08-29 , DOI: 10.1016/j.cej.2023.145726 Tengfei He , Baosheng Jin
|
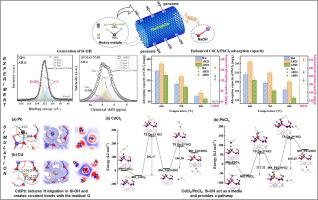
We attempted to activate the Si surface of Halloysite (Hal) to enhance its adsorption capacity for heavy metal (HM, CdCl2/PbCl2) vapors through thermal-alkali modification. A combination of experimental and simulation methods was used to analyze the stabilization mechanism of CdCl2/PbCl2 comprehensively. FTIR, 1H MAS NMR, and XPS results confirm the generation of Si-OH groups on the alkali-treated Hal (AHal) surface. The adsorption performance of adsorbents was investigated in a two-stage high-temperature adsorption setup, and the results show that a relatively short alkali treatment time (20 min) improved the adsorption capacity of Hal, while a longer treatment time (60 min) led to a negative impact. XPS results revealed that the adsorption of CdCl2/PbCl2 involved both physical and chemical adsorption. Grand Canonical Monte Carlo simulations demonstrated an increased adsorption potential of AHal and enhanced reactivity with the HMs. Density functional theory was employed to investigate the adsorption mechanism of Cd/Pb compounds on the Si surface. Compared to raw Hal, AHal exhibited higher adsorption energy for HMs after introducing Si-OH groups, resulting in a more stable configuration. The stabilization of Cd/Pb monomers on the Si surface primarily relies on promoting the H migration from Si-OH to interlayer O and then forming covalent bonds with the remaining active O atoms, while Si-OH played a role in facilitating the occurrence of De-HCl reaction, thereby transforming the adsorption of metal chlorides into more stable metal monomers adsorption.
中文翻译:

用于增强镉/铅蒸气捕获的碱改性埃洛石:通过实验和计算研究关注硅表面
我们尝试通过热碱改性来活化埃洛石(Hal)的Si表面,以增强其对重金属(HM、CdCl 2 /PbCl 2 )蒸气的吸附能力。采用实验与模拟相结合的方法,全面分析了CdCl 2 /PbCl 2的稳定机理。傅立叶变换红外光谱仪,11H MAS NMR 和 XPS 结果证实了碱处理的 Hal (AHal) 表面上生成了 Si-OH 基团。在两级高温吸附装置中研究了吸附剂的吸附性能,结果表明,相对较短的碱处理时间(20 min)提高了Hal的吸附能力,而较长的处理时间(60 min)则导致Hal的吸附能力下降。从而产生负面影响。XPS结果表明CdCl 2 /PbCl 2的吸附包括物理吸附和化学吸附。大正则蒙特卡罗模拟表明 AHal 的吸附潜力增加,并且与 HM 的反应性增强。采用密度泛函理论研究了Cd/Pb化合物在Si表面的吸附机理。与原始 Hal 相比,引入 Si-OH 基团后,AHal 对 HMs 表现出更高的吸附能,从而形成更稳定的构型。Si表面Cd/Pb单体的稳定主要依靠促进H从Si-OH迁移到层间O,然后与剩余的活性O原子形成共价键,而Si-OH则起到了促进De发生的作用。 -HCl反应,从而将金属氯化物的吸附转变为更稳定的金属单体的吸附。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号