Synthesis ( IF 2.2 ) Pub Date : 2023-08-28 , DOI: 10.1055/s-0041-1738451 Ever A. Blé González 1 , Roberto Martínez 2 , Diego Díaz Bautista 1 , Rosa María Chávez Santos 2 , María Teresa Ramírez Apán 2 , Miguel A. Vilchis Reyes 1
|
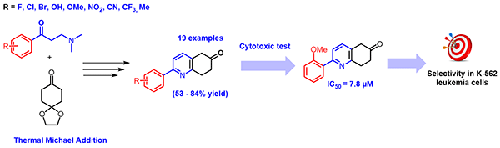
Herein we present a facile four-step synthetic method for the synthesis of novel 2-aryl-substituted 7,8-dihydroquinolin-6(5H)-ones as cytotoxic agents. The key step was the use of Mannich salts derived from acetophenones as a Michael acceptor in the reaction with cyclohexane-1,4-dione monoethylene acetal to give 1,5-dicarbonyl compounds that were treated with ammonium acetate to give the 7,8-dihydroquinolin-6(5H)-ones. The cytotoxic activity of the synthesized compounds was evaluated against seven cell lines. The observed data showed good selectivity for chronic myeloid leukemia line K-562. The synthetic route was simple and applicable to various functional group containing substrates. These types of compounds may be utilized as lead compounds in cancer research and drug discovery.
中文翻译:

2-芳基-7,8-二氢喹啉-6(5H)-酮的合成及细胞毒性评价
在此,我们提出了一种简便的四步合成方法,用于合成新型2-芳基取代的7,8-二氢喹啉-6(5 H )-酮作为细胞毒剂。关键步骤是使用苯乙酮衍生的曼尼希盐作为迈克尔受体,与环己烷-1,4-二酮单乙烯缩醛反应,得到1,5-二羰基化合物,用乙酸铵处理,得到7,8-二羰基化合物。二氢喹啉-6(5 H )-酮。针对七种细胞系评估了合成化合物的细胞毒活性。观察数据显示对慢性粒细胞白血病株K-562具有良好的选择性。该合成路线简单,适用于各种含官能团的底物。这些类型的化合物可用作癌症研究和药物发现中的先导化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号