Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2023-08-28 , DOI: 10.1016/j.bmc.2023.117453 Nolan Chatron 1 , Manon Boulven 2 , Adrien Montagut-Romans 2 , Flavien Ponsot 3 , Maïwenn Jacolot 3 , Hervé Caruel 4 , Etienne Benoît 1 , Florence Popowycz 3 , Virginie Lattard 1
|
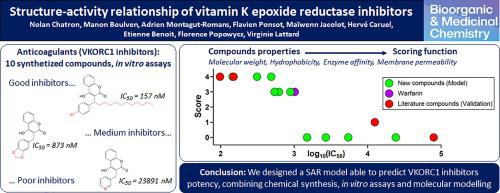
Vitamin K antagonists (VKAs) anticoagulants have been used since the 1950s as medicines and rodenticides. These molecules are mainly 4-hydroxycoumarin derivatives and act by inhibiting the vitamin K epoxide reductase (VKORC1), an endoplasmic reticulum membrane resident enzyme. However, many VKORC1 mutations have been reported over the last decade, inducing VKAs resistances and thus treatments failures. Although studies have reported experimental and computational investigations of VKAs based on VKORC1 structural homology models, the development of new effective anticoagulants has been quite complex due to the lack of structural data and reliable structure–activity relationships. However, the recent publication of VKORC1 crystal structure provides new information for further studies. Based on these findings, we combined chemical synthesis, enzymatic assays and molecular modelling methods to design a structure–activity relationship (SAR) model. Our results proved that the lipophilicity, the membrane permeability of inhibitors and their affinity towards human VKORC1 enzyme are the main characteristics for potent anticoagulants. Our SAR model managed to rank compounds according to their ability to inhibit the human VKORC1. Such a tool might constitute an alternative to evaluate new molecules potency before their chemical synthesis and biological assessment and might assist the development of new VKAs.
中文翻译:

结合新化合物的化学合成、酶分析和分子建模,设计维生素 K 环氧化物还原酶 (VKORC1) 抑制剂的构效关系模型
自 20 世纪 50 年代以来,维生素 K 拮抗剂 (VKA)抗凝剂一直被用作药物和杀鼠剂。这些分子主要是 4-羟基香豆素衍生物,通过抑制维生素 K 环氧化物还原酶 (VKORC1)(一种内质网膜驻留酶)发挥作用。然而,过去十年中报道了许多 VKORC1 突变,导致 VKA 耐药,从而导致治疗失败。尽管有研究报道了基于 VKORC1 结构同源模型的 VKA 的实验和计算研究,但由于缺乏结构数据和可靠的构效关系,新型有效抗凝剂的开发相当复杂。然而,最近发表的VKORC1晶体结构为进一步研究提供了新的信息。基于这些发现,我们结合化学合成、酶分析和分子建模方法设计了构效关系(SAR)模型。我们的结果证明,抑制剂的亲脂性、膜渗透性及其对人 VKORC1 酶的亲和力是有效抗凝剂的主要特征。我们的 SAR 模型设法根据化合物抑制人类 VKORC1 的能力对化合物进行排名。这样的工具可能构成在化学合成和生物学评估之前评估新分子效力的替代方案,并可能有助于新 VKA 的开发。

































 京公网安备 11010802027423号
京公网安备 11010802027423号