当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organic Matter Counteracts the Enhancement of Cr(III) Extractability during the Fe(II)-Catalyzed Ferrihydrite Transformation: A Nanoscale- and Molecular-Level Investigation
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-08-28 , DOI: 10.1021/acs.est.3c03848 Xing Xia 1, 2 , Jin Liu 3 , Lin Jin 2 , Jian Wang 4 , Aminu Inuwa Darma 2 , Chao He 2 , Mohsen Shakouri 4 , Yongfeng Hu 4 , Jianjun Yang 5
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-08-28 , DOI: 10.1021/acs.est.3c03848 Xing Xia 1, 2 , Jin Liu 3 , Lin Jin 2 , Jian Wang 4 , Aminu Inuwa Darma 2 , Chao He 2 , Mohsen Shakouri 4 , Yongfeng Hu 4 , Jianjun Yang 5
Affiliation
|
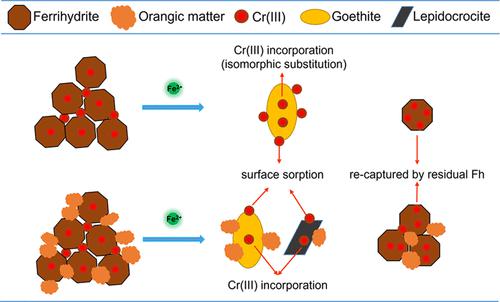
Phase transformation of ferrihydrite to more stable Fe (oxyhydr)oxides, catalyzed by iron(II) [Fe(II)], significantly influences the mobility of heavy metals [e.g., chromium (Cr)] associated with ferrihydrite. However, the impact of organic matter (OM) on the behavior of Cr(III) in the Fe(II)-catalyzed transformation of ferrihydrite and the underlying mechanisms are unclear. Here, the Fe(II)-catalyzed transformation of the coprecipitates of Fe(III), Cr(III), or rice straw-derived OM was studied at the nanoscale and molecular levels using Fe and Cr K-edge X-ray absorption spectroscopy and spherical aberration corrected scanning transmission electron microscopy (Cs-STEM). Batch extraction results suggested that the OM counteracted the enhancement of Cr(III) extractability during the Fe(II)-catalyzed transformation. Cs-STEM and XAS analysis suggested that Cr(III) could be incorporated into the goethite formed by Fe(II)-catalyzed ferrihydrite transformation, which, however, was inhibited by the OM. Furthermore, Cs-STEM analysis also provided direct nanoscale level evidence that residual ferrihydrite could re-immobilize the released Cr(III) during the Fe(II)-catalyzed transformation process. These results highlighted that the decreased extractability of Cr(III) mainly resulted from the inhibition of OM on the Fe(II)-catalyzed transformation of ferrihydrite to secondary Fe (oxyhydr)oxides, which facilitates insightful understanding and prediction of the geochemical cycling of Cr in soils with active redox dynamics.
中文翻译:

有机物抵消 Fe(II) 催化水铁矿转化过程中 Cr(III) 萃取能力的增强:纳米级和分子级研究
在铁(II) [Fe(II)]的催化下,水铁矿向更稳定的Fe(羟基)氧化物的相变显着影响与水铁矿相关的重金属[例如铬(Cr)]的迁移率。然而,有机物 (OM) 对 Fe(II) 催化水铁矿转化中 Cr(III) 行为的影响及其潜在机制尚不清楚。在这里,使用 Fe 和 Cr K 边 X 射线吸收光谱在纳米级和分子水平上研究了 Fe(III)、Cr(III) 或稻草衍生的 OM 共沉淀物的 Fe(II) 催化转化和球差校正扫描透射电子显微镜(Cs-STEM)。批量萃取结果表明,OM 抵消了 Fe(II) 催化转化过程中 Cr(III) 萃取率的提高。Cs-STEM 和 XAS 分析表明,Cr(III) 可以并入由 Fe(II) 催化的水铁矿转化形成的针铁矿中,然而,这被 OM 抑制。此外,Cs-STEM 分析还提供了直接的纳米级证据,表明残留的水铁矿可以在 Fe(II) 催化转化过程中重新固定释放的 Cr(III)。这些结果表明,Cr(III)的萃取率下降主要是由于OM抑制了Fe(II)催化的水铁矿向次生Fe(羟基)氧化物的转化,这有助于深入了解和预测Cr的地球化学循环在具有活跃氧化还原动力学的土壤中。
更新日期:2023-08-28
中文翻译:

有机物抵消 Fe(II) 催化水铁矿转化过程中 Cr(III) 萃取能力的增强:纳米级和分子级研究
在铁(II) [Fe(II)]的催化下,水铁矿向更稳定的Fe(羟基)氧化物的相变显着影响与水铁矿相关的重金属[例如铬(Cr)]的迁移率。然而,有机物 (OM) 对 Fe(II) 催化水铁矿转化中 Cr(III) 行为的影响及其潜在机制尚不清楚。在这里,使用 Fe 和 Cr K 边 X 射线吸收光谱在纳米级和分子水平上研究了 Fe(III)、Cr(III) 或稻草衍生的 OM 共沉淀物的 Fe(II) 催化转化和球差校正扫描透射电子显微镜(Cs-STEM)。批量萃取结果表明,OM 抵消了 Fe(II) 催化转化过程中 Cr(III) 萃取率的提高。Cs-STEM 和 XAS 分析表明,Cr(III) 可以并入由 Fe(II) 催化的水铁矿转化形成的针铁矿中,然而,这被 OM 抑制。此外,Cs-STEM 分析还提供了直接的纳米级证据,表明残留的水铁矿可以在 Fe(II) 催化转化过程中重新固定释放的 Cr(III)。这些结果表明,Cr(III)的萃取率下降主要是由于OM抑制了Fe(II)催化的水铁矿向次生Fe(羟基)氧化物的转化,这有助于深入了解和预测Cr的地球化学循环在具有活跃氧化还原动力学的土壤中。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号