Molecular Catalysis ( IF 3.9 ) Pub Date : 2023-08-26 , DOI: 10.1016/j.mcat.2023.113493 Yu Zhang , Sheng-Nan Du , Mia Guo , Xiao Meng Wu , Yong Yi Yu , Feng-Shou Liu , Chang Xu
|
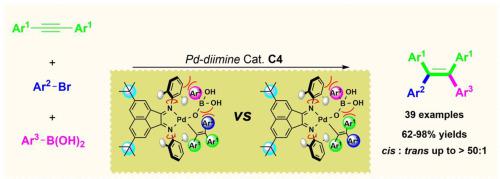
Herein, a novel palladium α-diimine complex, in which two bulky tert-butyl substituents were incorporated into the acenaphthyl backbone of diimine, catalyzed carbofunctionalization of internal aklynes protocol has been established for the highly regio- and stereo-selective synthesis of cis-type tetraarylethylenes (TAEs). The structure-activity relationship of the catalyst structures and their catalytic properties was also well investigated. The experimental results demonstrated that introducing of two bulky substituents into the conjugated acenaphthyl framework of the diimine is the crucial reason for the excellent reactivities and cis-selectivities. It not only delivered the desired cis-TAE products in up to 50:1 cis/trans isomeric ratio and significantly expanded the substrate scope, including the electron-deficient aryl halides and substrates with bulky substitutions, to streamline the synthesis of cis-TAEs, which had been challenging to prepare according to the conventional carbo-metalation methods.
中文翻译:

钯α-二亚胺配合物催化内部炔烃的高效顺式选择性碳官能化
在此,建立了一种新型的钯α-二亚胺络合物,其中两个大的叔丁基取代基被纳入二亚胺的苊基主链中,并催化了内部aklynes的碳功能化方案,用于顺式-型的高度区域和立体选择性合成四芳基乙烯(TAE)。催化剂结构的构效关系及其催化性能也得到了很好的研究。实验结果表明,在二亚胺的共轭苊基骨架中引入两个大的取代基是其优异的反应活性和顺式选择性的关键原因。它不仅以高达 50:1 的cis提供所需的cis -TAE 产品/反式异构体比例并显着扩大了底物范围,包括缺电子芳基卤化物和具有大量取代基的底物,以简化顺式-TAE的合成,这对于根据传统碳金属化方法制备具有挑战性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号