Cellular Signalling ( IF 4.4 ) Pub Date : 2023-08-26 , DOI: 10.1016/j.cellsig.2023.110876 Sayanta Dutta 1 , Sushweta Mahalanobish 1 , Sukanya Saha 1 , Mullicka Mandal 2 , Sanchari Begam 2 , Pritam Sadhukhan 1 , Sumit Ghosh 1 , Goutam Brahmachari 2 , Parames C Sil 1
|
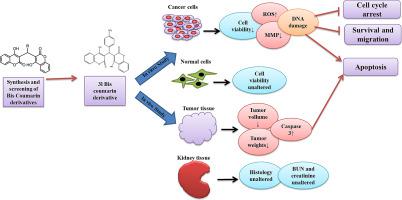
Selective initiation of programmed cell death in cancer cells than normal cells is reflected as an attractive chemotherapeutic strategy. In the current study, a series of synthetic bis-coumarin derivatives were synthesized possessing reactive oxygen species (ROS) modulating functional groups and examined in four cancerous and two normal cell lines for their cytotoxic ability using MTT assay. Among these compounds, 3 l emerged as the most promising derivative in persuading apoptosis in human renal carcinoma cells (SKRC-45) among diverse cancer cell lines. 3 l causes significantly less cytotoxicity to normal kidney cells compared to cisplatin. This compound was able to induce apoptosis and cell-cycle arrest by modulating the p53 mediated apoptotic pathways via the generation of ROS, decreasing mitochondrial membrane potential, and causing DNA fragmentation. Unlike cisplatin, the 3 l derivative was found to inhibit the nuclear localisation of NF-κB in SKRC-45 cells. It was also found to reduce the proliferation, survival and migration ability of SKRC-45 cells by downregulating COX-2/ PTGES2 cascade and MMP-2. In an in vivo tumor model, 3 l showed an anticancer effect by reducing the mean tumor mass, volume and inducing caspase-3 activation, without affecting kidney function. Further studies are needed to establish 3 l as a promising anti-cancer drug candidate.
中文翻译:

通过选择性诱导活性氧介导的细胞凋亡,对新型 3,3'-((4-硝基苯基)亚甲基)双(4-羟基-2H-chromen-2-one) 衍生物作为潜在抗癌剂的生物学评价
与正常细胞相比,癌细胞选择性启动程序性细胞死亡被认为是一种有吸引力的化疗策略。在当前的研究中,合成了一系列具有活性氧(ROS)调节功能基团的合成双香豆素衍生物,并使用MTT测定在四种癌细胞系和两种正常细胞系中检查了它们的细胞毒性能力。在这些化合物中,3 l 成为最有希望促进不同癌细胞系中人肾癌细胞 (SKRC-45) 凋亡的衍生物。与顺铂相比,3 l 对正常肾细胞的细胞毒性显着降低。该化合物能够通过产生 ROS、降低线粒体膜电位并引起 DNA 断裂来调节 p53 介导的细胞凋亡途径,从而诱导细胞凋亡和细胞周期停滞。与顺铂不同,3L 衍生物被发现可抑制 SKRC-45 细胞中 NF-κB 的核定位。还发现它通过下调 COX-2/PTGES2 级联和 MMP-2 来降低 SKRC-45 细胞的增殖、存活和迁移能力。在体内肿瘤模型中,3 l 通过减少平均肿瘤质量、体积和诱导 caspase-3 激活而显示出抗癌作用,且不影响肾功能。需要进一步的研究来确定 3 l 作为有前途的抗癌候选药物。































 京公网安备 11010802027423号
京公网安备 11010802027423号