当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spatial and electronic effects synergistically enhanced electrocatalytic oxygen evolution using atomic iridium-anchored cobalt oxyhydroxide nanosheets
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-08-25 , DOI: 10.1016/j.apcatb.2023.123227 Bo Yang , Meiqian Li , Zhirong Zhang , Shaoqing Chen , Miaomiao Wang , Li Sheng , Libo Deng , Rui Si , Maohong Fan , Huihuang Chen
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-08-25 , DOI: 10.1016/j.apcatb.2023.123227 Bo Yang , Meiqian Li , Zhirong Zhang , Shaoqing Chen , Miaomiao Wang , Li Sheng , Libo Deng , Rui Si , Maohong Fan , Huihuang Chen
|
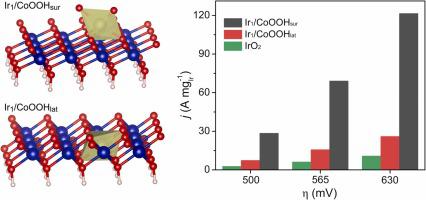
Single-atom catalysts (SACs) based on noble metals play irreplaceable roles in the field of catalysis but unfortunately suffer from spatial constraints ranging from either lattice substitution, atomic dilution, or in-layer immobilization. Herein, this work was designed to overcome the limitation by locating discrete dangling coordinatively unsaturated [IrO] motif atop γ-phase cobalt oxyhydroxide (γ-CoOOH) nanosheets to create a spatially novel catalyst (Ir/CoOOH). For comparison, the lattice-doped catalyst (Ir/CoOOH) was also synthesized via substituting Co in γ-CoOOH by Ir single atoms. The distinct location arrangements of Ir single atoms generated two different active sites that both weakened the adsorption of oxygenated intermediates relative to γ-CoOOH due to the upshifted O 2-band center. Moreover, Ir/CoOOH displayed a much weaker adsorption capability (closer to an ideal catalyst) than Ir/CoOOH. Therefore, the spatial and electronic effects of discrete dangling [IrO] motifs atop Ir/CoOOH synergistically optimized the adsorption of oxygenated intermediates and thus gained the lowest energy barrier of the rate-determining step for oxygen evolution reaction. When used as the cathode catalyst in rechargeable zinc-air batteries, Ir/CoOOH exhibited higher power density (101 mW cm) and cycling durability (800 h) than IrO (94 mW cm, 50 h). This study broadens noble metal-based SACs analogues and offers appealing opportunity to design target catalysts with the synergism of spatial and electronic effects for diverse catalytic applications.
中文翻译:

使用原子铱锚定羟基氧化钴纳米片,空间和电子效应协同增强电催化析氧
基于贵金属的单原子催化剂(SAC)在催化领域发挥着不可替代的作用,但不幸的是受到晶格取代、原子稀释或层内固定等空间限制。在此,这项工作旨在通过将离散的悬空配位不饱和[IrO]基序定位在γ相羟基氧化钴(γ-CoOOH)纳米片上来克服这一限制,以创建一种空间新颖的催化剂(Ir/CoOOH)。为了进行比较,还通过用 Ir 单原子取代 γ-CoOOH 中的 Co 合成了晶格掺杂催化剂 (Ir/CoOOH)。 Ir单原子的不同位置排列产生了两个不同的活性位点,由于O 2 带中心的上移,这两个活性位点都削弱了相对于γ-CoOOH的含氧中间体的吸附。此外,Ir/CoOOH 的吸附能力比 Ir/CoOOH 弱得多(更接近理想催化剂)。因此,Ir/CoOOH顶部离散悬空[IrO]基序的空间和电子效应协同优化了含氧中间体的吸附,从而获得了析氧反应速率决定步骤的最低能垒。当用作可充电锌空气电池的阴极催化剂时,Ir/CoOOH表现出比IrO(94 mW cm,50 h)更高的功率密度(101 mW cm)和循环耐久性(800 h)。这项研究拓宽了基于贵金属的 SAC 类似物,并为设计具有空间和电子效应协同作用的目标催化剂提供了诱人的机会,适用于各种催化应用。
更新日期:2023-08-25
中文翻译:

使用原子铱锚定羟基氧化钴纳米片,空间和电子效应协同增强电催化析氧
基于贵金属的单原子催化剂(SAC)在催化领域发挥着不可替代的作用,但不幸的是受到晶格取代、原子稀释或层内固定等空间限制。在此,这项工作旨在通过将离散的悬空配位不饱和[IrO]基序定位在γ相羟基氧化钴(γ-CoOOH)纳米片上来克服这一限制,以创建一种空间新颖的催化剂(Ir/CoOOH)。为了进行比较,还通过用 Ir 单原子取代 γ-CoOOH 中的 Co 合成了晶格掺杂催化剂 (Ir/CoOOH)。 Ir单原子的不同位置排列产生了两个不同的活性位点,由于O 2 带中心的上移,这两个活性位点都削弱了相对于γ-CoOOH的含氧中间体的吸附。此外,Ir/CoOOH 的吸附能力比 Ir/CoOOH 弱得多(更接近理想催化剂)。因此,Ir/CoOOH顶部离散悬空[IrO]基序的空间和电子效应协同优化了含氧中间体的吸附,从而获得了析氧反应速率决定步骤的最低能垒。当用作可充电锌空气电池的阴极催化剂时,Ir/CoOOH表现出比IrO(94 mW cm,50 h)更高的功率密度(101 mW cm)和循环耐久性(800 h)。这项研究拓宽了基于贵金属的 SAC 类似物,并为设计具有空间和电子效应协同作用的目标催化剂提供了诱人的机会,适用于各种催化应用。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号