当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Imidazo[1,2-a]pyridine-Fused 1,3-Benzodiazepine Derivatives with Anticancer Activity via a One-Pot Cascade GBB-3CR/Pd(II)-Catalyzed Azide-Isocyanide Coupling/Cyclization Process
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-08-24 , DOI: 10.1021/acs.joc.3c01341 Cheng-Ran Zhong 1 , Yang-Hong Zhang 1 , Gang Yao 1 , Hai-Li Zhu 2 , Yin-Di Hu 2 , Zhi-Gang Zeng 3 , Chang-Zhou Liao 3 , Hui-Ting He 1 , Ya-Ting Luo 3 , Jun Xiong 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-08-24 , DOI: 10.1021/acs.joc.3c01341 Cheng-Ran Zhong 1 , Yang-Hong Zhang 1 , Gang Yao 1 , Hai-Li Zhu 2 , Yin-Di Hu 2 , Zhi-Gang Zeng 3 , Chang-Zhou Liao 3 , Hui-Ting He 1 , Ya-Ting Luo 3 , Jun Xiong 1
Affiliation
|
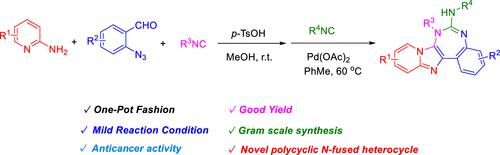
A new one-pot synthesis of imidazo[1,2-a]pyridine-fused 1,3-benzodiazepine derivatives via a sequential GBB-3CR/Pd(II)-catalyzed azide-isocyanide coupling/cyclization process was developed. The Groebke–Blackburn–Bienaymé three-component reactions (GBB-3CR) of 2-aminopyridine, 2-azidobenzaldehydes, and isocyanides in the presence of a catalytic amount of p-toluenesulfonic acid gave azide intermediates without separation. The reaction was followed by using another molecule of isocyanides to produce imidazo[1,2-a]pyridine-fused 1,3-benzodiazepine derivatives in good yields by the Pd(II)-catalyzed azide-isocyanide coupling/cyclization reaction. The synthetic approach produces novel nitrogen-fused polycyclic heterocycles under mild reaction conditions. The preliminary biological evaluation demonstrated that compound 6a inhibited glioma cells efficiently, suggesting potentially broad applications of the approach for synthesis and medicinal chemistry.
中文翻译:

通过一锅级联 GBB-3CR/Pd(II) 催化叠氮化物-异氰化物偶联/环化过程合成具有抗癌活性的咪唑并[1,2-a]吡啶稠合 1,3-苯二氮卓衍生物
开发了一种通过连续 GBB-3CR/Pd(II) 催化叠氮化物-异氰化物偶联/环化工艺一锅合成咪唑并[1,2- a ]吡啶稠合 1,3-苯二氮卓衍生物的新方法。在催化量的对甲苯磺酸存在下,2-氨基吡啶、2-叠氮基苯甲醛和异氰化物的 Groebke-Blackburn-Bienaymé 三组分反应 (GBB-3CR) 无需分离即可得到叠氮化物中间体。随后使用另一分子异氰化物通过Pd(II) 催化的叠氮化物-异氰化物偶联/环化反应以良好的产率生产咪唑并[1,2- a ]吡啶稠合的 1,3-苯二氮卓衍生物。该合成方法在温和的反应条件下产生新型氮稠合多环杂环。初步生物学评估表明,化合物6a能有效抑制神经胶质瘤细胞,表明该方法在合成和药物化学方面具有广泛的应用前景。
更新日期:2023-08-24
中文翻译:

通过一锅级联 GBB-3CR/Pd(II) 催化叠氮化物-异氰化物偶联/环化过程合成具有抗癌活性的咪唑并[1,2-a]吡啶稠合 1,3-苯二氮卓衍生物
开发了一种通过连续 GBB-3CR/Pd(II) 催化叠氮化物-异氰化物偶联/环化工艺一锅合成咪唑并[1,2- a ]吡啶稠合 1,3-苯二氮卓衍生物的新方法。在催化量的对甲苯磺酸存在下,2-氨基吡啶、2-叠氮基苯甲醛和异氰化物的 Groebke-Blackburn-Bienaymé 三组分反应 (GBB-3CR) 无需分离即可得到叠氮化物中间体。随后使用另一分子异氰化物通过Pd(II) 催化的叠氮化物-异氰化物偶联/环化反应以良好的产率生产咪唑并[1,2- a ]吡啶稠合的 1,3-苯二氮卓衍生物。该合成方法在温和的反应条件下产生新型氮稠合多环杂环。初步生物学评估表明,化合物6a能有效抑制神经胶质瘤细胞,表明该方法在合成和药物化学方面具有广泛的应用前景。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号