当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Relationship between Ionic Conductivity and Solvation Structures of Localized High-Concentration Fluorinated Electrolytes for Lithium-Ion Batteries
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-08-22 , DOI: 10.1021/acs.jpclett.3c01453 Md Jamil Hossain 1 , Qisheng Wu 1 , Edelmy J Marin Bernardez 2, 3 , Calvin D Quilty 2, 3 , Amy C Marschilok 2, 3, 4, 5 , Esther S Takeuchi 2, 3, 4, 5 , David C Bock 3, 4 , Kenneth J Takeuchi 2, 3, 4, 5 , Yue Qi 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-08-22 , DOI: 10.1021/acs.jpclett.3c01453 Md Jamil Hossain 1 , Qisheng Wu 1 , Edelmy J Marin Bernardez 2, 3 , Calvin D Quilty 2, 3 , Amy C Marschilok 2, 3, 4, 5 , Esther S Takeuchi 2, 3, 4, 5 , David C Bock 3, 4 , Kenneth J Takeuchi 2, 3, 4, 5 , Yue Qi 1
Affiliation
|
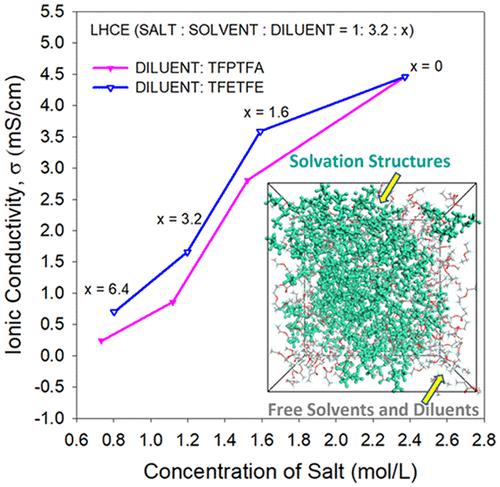
Localized high-concentration electrolytes (LHCEs) combine a diluent with a high-concentration electrolyte, offering promising properties. The ions, solvent, and diluent interact to form complex heterogeneous liquid structures, where high salt concentration clusters are embedded in the diluent. Optimizing LHCEs for desired electrolyte properties like high ionic conductivity, low viscosity, and effective solid electrolyte interphase (SEI) formation ability within the vast chemical and compositional design space requires deeper understanding and theoretical guidance. We investigated the structures and conductivities of LHCEs based on a fluorinated solvent with two different diluents at varying concentrations. 2,2,3,3-Tetrafluoropropyl trifluoroacetate (TFPTFA) enters the solvation cluster due to its stronger Li-ion interactions, whereas 1,1,2,2-tetrafluoroethyl 2,2,2-trifluoroethyl ether (TFETFE) enters only at extremely high diluent concentrations. The ionic conductivity increases with decreasing diluent concentrations, with a slope change during cluster percolation. Overall, TFETFE demonstrates higher effectiveness than TFPTFA, forming higher local salt concentration clusters and resulting in higher ionic conductivity.
中文翻译:

锂离子电池局部高浓度氟化电解质的离子电导率与溶剂化结构的关系
局部高浓度电解质(LHCE)将稀释剂与高浓度电解质相结合,具有前景广阔的特性。离子、溶剂和稀释剂相互作用形成复杂的异质液体结构,其中高盐浓度簇嵌入稀释剂中。在广阔的化学和成分设计空间内优化 LHCE 以获得所需的电解质特性,如高离子电导率、低粘度和有效的固体电解质界面 (SEI) 形成能力,需要更深入的理解和理论指导。我们研究了基于含不同浓度的两种不同稀释剂的氟化溶剂的 LHCE 的结构和电导率。2,2,3,3-四氟丙基三氟乙酸酯 (TFPTFA) 由于其更强的锂离子相互作用而进入溶剂化簇,而 1,1,2,2-四氟乙基 2,2,2-三氟乙基醚 (TFETFE) 仅在极高的稀释浓度。离子电导率随着稀释剂浓度的降低而增加,在簇渗滤过程中斜率发生变化。总体而言,TFETFE 表现出比 TFPTFA 更高的有效性,形成更高的局部盐浓度簇并导致更高的离子电导率。
更新日期:2023-08-22
中文翻译:

锂离子电池局部高浓度氟化电解质的离子电导率与溶剂化结构的关系
局部高浓度电解质(LHCE)将稀释剂与高浓度电解质相结合,具有前景广阔的特性。离子、溶剂和稀释剂相互作用形成复杂的异质液体结构,其中高盐浓度簇嵌入稀释剂中。在广阔的化学和成分设计空间内优化 LHCE 以获得所需的电解质特性,如高离子电导率、低粘度和有效的固体电解质界面 (SEI) 形成能力,需要更深入的理解和理论指导。我们研究了基于含不同浓度的两种不同稀释剂的氟化溶剂的 LHCE 的结构和电导率。2,2,3,3-四氟丙基三氟乙酸酯 (TFPTFA) 由于其更强的锂离子相互作用而进入溶剂化簇,而 1,1,2,2-四氟乙基 2,2,2-三氟乙基醚 (TFETFE) 仅在极高的稀释浓度。离子电导率随着稀释剂浓度的降低而增加,在簇渗滤过程中斜率发生变化。总体而言,TFETFE 表现出比 TFPTFA 更高的有效性,形成更高的局部盐浓度簇并导致更高的离子电导率。







































 京公网安备 11010802027423号
京公网安备 11010802027423号