当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Naphthalimide Derivatives and Their Luminescence upon Complexation with Cucurbit[n]uril Hosts
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-08-23 , DOI: 10.1021/acs.joc.3c01111
Yong Wu 1 , Dongdong Sun 1 , Xie Han 1, 2 , Zhiyong Zhao 1, 2 , Feng Liang 1, 2 , Simin Liu 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-08-23 , DOI: 10.1021/acs.joc.3c01111
Yong Wu 1 , Dongdong Sun 1 , Xie Han 1, 2 , Zhiyong Zhao 1, 2 , Feng Liang 1, 2 , Simin Liu 1, 2
Affiliation
|
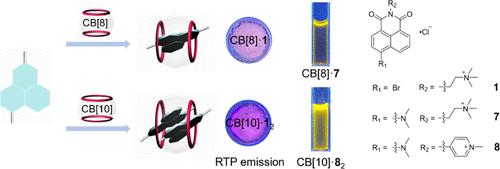
A series of naphthalimide derivatives are synthesized and their binding behavior upon complexation with cucurbit[n]urils (CB[n]s) has been investigated. With a heavy atom (bromine) on the naphthalimide core, 4-bromo-1,8-naphthalimide derivatives 1–4 show short room-temperature phosphorescence (RTP) lifetimes with low quantum yields. Their RTP properties are significantly enhanced in the presence of CB[8] or CB[10] both in aqueous solution and solid state owing to the efficient suppression of nonradiative decay and isolation of quenching factors by the rigid cavity of CB[n]. Without the bromine atom, 1,8-naphthalimide derivatives 5 and 6 show strong excimer emission upon complexation with CB[10] accompanied by fluorescence transition from blue to cyan. The fluorescence colors of 4-(dimethylamino)-1,8-naphthalimide derivatives 7 and 8 change from blue to white to yellow with the addition of CB[n]. This host–guest complexation strategy to modulate the luminescence of the luminophore would further broaden the application of luminescent materials.
中文翻译:

萘二甲酰亚胺衍生物的合成及其与葫芦[n]脲主体络合后的发光
合成了一系列萘酰亚胺衍生物,并研究了它们与葫芦[ n ]脲(CB[ n ]s)络合时的结合行为。萘二甲酰亚胺核心上有一个重原子(溴),4-溴-1,8-萘二甲酰亚胺衍生物1 – 4表现出较短的室温磷光 (RTP) 寿命和较低的量子产率。由于 CB[ n ] 的刚性空腔有效抑制非辐射衰变和隔离猝灭因子,在水溶液和固态中存在 CB[8] 或 CB[10] 时,它们的 RTP 性能显着增强。没有溴原子,1,8-萘二甲酰亚胺衍生物5和6在与 CB[10] 络合时表现出强烈的准分子发射,并伴随着从蓝色到青色的荧光转变。随着CB[ n ]的加入,4-(二甲氨基)-1,8-萘二甲酰亚胺衍生物7和8的荧光颜色从蓝色变为白色再变为黄色。这种调节发光体发光的主客体络合策略将进一步拓宽发光材料的应用。
更新日期:2023-08-23
中文翻译:

萘二甲酰亚胺衍生物的合成及其与葫芦[n]脲主体络合后的发光
合成了一系列萘酰亚胺衍生物,并研究了它们与葫芦[ n ]脲(CB[ n ]s)络合时的结合行为。萘二甲酰亚胺核心上有一个重原子(溴),4-溴-1,8-萘二甲酰亚胺衍生物1 – 4表现出较短的室温磷光 (RTP) 寿命和较低的量子产率。由于 CB[ n ] 的刚性空腔有效抑制非辐射衰变和隔离猝灭因子,在水溶液和固态中存在 CB[8] 或 CB[10] 时,它们的 RTP 性能显着增强。没有溴原子,1,8-萘二甲酰亚胺衍生物5和6在与 CB[10] 络合时表现出强烈的准分子发射,并伴随着从蓝色到青色的荧光转变。随着CB[ n ]的加入,4-(二甲氨基)-1,8-萘二甲酰亚胺衍生物7和8的荧光颜色从蓝色变为白色再变为黄色。这种调节发光体发光的主客体络合策略将进一步拓宽发光材料的应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号