当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Digital SERS Protocol Using Au Nanoparticle-Based Extrinsic Raman Labels for the Determination of SARS-CoV-2 Spike Protein in Saliva Samples
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2023-08-23 , DOI: 10.1021/acsanm.3c01979 Ariadne Tuckmantel Bido 1 , Alexandre G. Brolo 1, 2
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2023-08-23 , DOI: 10.1021/acsanm.3c01979 Ariadne Tuckmantel Bido 1 , Alexandre G. Brolo 1, 2
Affiliation
|
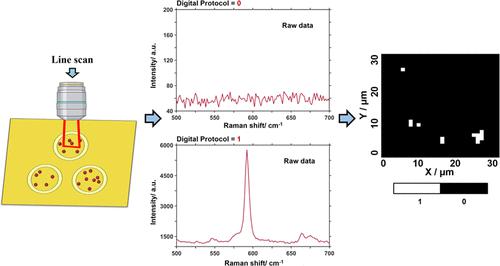
Surface-enhanced Raman scattering (SERS)-based immunoassays have several advantages, such as high sensitivity and multiplex capabilities; they are emerging as a potential avenue for early disease diagnosis and screening. Here, we focused on SERS-based heterogeneous immunoassays, in which the number of extrinsic Raman labels (ERLs) at the sensor surface is related to the concentration of the intended target. The ERLs are made of gold nanoparticles and are constructed to be selective to the target and to boost the signal of a Raman reporter. However, as the concentration of the target biomarker decreases, the number of ERLs per unit of area (mm2) also decreases, leading to a small number of ERLs being probed within an exciting laser spot. This poor sampling adds to the large intensity variations inherent to the SERS effect, resulting in a loss in the linearity between the SERS signal and the marker/target analyte concentration. This characteristic has rendered SERS-based immunoassays unreliable for quantification at low bioanalyte concentrations. We propose the use of a digital quantification protocol to overcome this problem. A SERS-based sandwich immunoassay was developed for the detection of the SARS-CoV-2 S1–S2 spike protein in saliva. A conventional data analysis that relates SERS intensities to concentration was compared to the digital protocol for the same dataset. The digital SERS assay presented an LOD of 6.3 ng·mL–1 or 34.9 pM and an LOQ of 19.0 ng·mL–1 or 105.7 pM within a 95% confidence level. These metrics show an 11-fold improvement compared to the conventional data analysis.
中文翻译:

使用基于金纳米粒子的外在拉曼标记的数字 SERS 协议测定唾液样本中的 SARS-CoV-2 刺突蛋白
基于表面增强拉曼散射(SERS)的免疫测定具有多种优点,例如高灵敏度和多重功能;它们正在成为早期疾病诊断和筛查的潜在途径。在这里,我们重点关注基于 SERS 的异质免疫测定,其中传感器表面的外在拉曼标签 (ERL) 数量与预期目标的浓度相关。ERL 由金纳米颗粒制成,旨在对目标具有选择性并增强拉曼报告基因的信号。然而,随着目标生物标志物浓度的降低,每单位面积 (mm 2)也会减少,导致在令人兴奋的激光点内探测到少量的 ERL。这种不良采样增加了 SERS 效应固有的大强度变化,导致 SERS 信号和标记/目标分析物浓度之间的线性损失。这一特性使得基于 SERS 的免疫测定在低生物分析物浓度下的定量不可靠。我们建议使用数字量化协议来克服这个问题。开发了一种基于 SERS 的夹心免疫测定法,用于检测唾液中的 SARS-CoV-2 S1-S2 刺突蛋白。将 SERS 强度与浓度相关的传统数据分析与同一数据集的数字协议进行了比较。数字 SERS 检测的 LOD 为 6.3 ng·mL –1或 34.9 pM,LOQ 为 19.0 ng·mL –1或 105.7 pM,置信水平为 95%。与传统数据分析相比,这些指标显示出 11 倍的改进。
更新日期:2023-08-23
中文翻译:

使用基于金纳米粒子的外在拉曼标记的数字 SERS 协议测定唾液样本中的 SARS-CoV-2 刺突蛋白
基于表面增强拉曼散射(SERS)的免疫测定具有多种优点,例如高灵敏度和多重功能;它们正在成为早期疾病诊断和筛查的潜在途径。在这里,我们重点关注基于 SERS 的异质免疫测定,其中传感器表面的外在拉曼标签 (ERL) 数量与预期目标的浓度相关。ERL 由金纳米颗粒制成,旨在对目标具有选择性并增强拉曼报告基因的信号。然而,随着目标生物标志物浓度的降低,每单位面积 (mm 2)也会减少,导致在令人兴奋的激光点内探测到少量的 ERL。这种不良采样增加了 SERS 效应固有的大强度变化,导致 SERS 信号和标记/目标分析物浓度之间的线性损失。这一特性使得基于 SERS 的免疫测定在低生物分析物浓度下的定量不可靠。我们建议使用数字量化协议来克服这个问题。开发了一种基于 SERS 的夹心免疫测定法,用于检测唾液中的 SARS-CoV-2 S1-S2 刺突蛋白。将 SERS 强度与浓度相关的传统数据分析与同一数据集的数字协议进行了比较。数字 SERS 检测的 LOD 为 6.3 ng·mL –1或 34.9 pM,LOQ 为 19.0 ng·mL –1或 105.7 pM,置信水平为 95%。与传统数据分析相比,这些指标显示出 11 倍的改进。































 京公网安备 11010802027423号
京公网安备 11010802027423号