Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-08-23 , DOI: 10.1016/j.molliq.2023.122788 Chunjuan Huang , Ganbing Yao
|
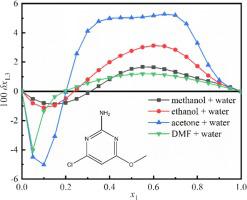
Equilibrium mole fraction solubility of 2-amino-4-chloro-6-methoxypyrimidine in methanol/ethanol/acetone/N,N-Dimethylformamide (DMF) plus water was experimentally measured by isothermal saturation method at 278.15–323.15 K under 101.2 kPa. The mixed solvents with low moisture and high temperature played a supportive role in the dissolution of 2-amino-4-chloro-6-methoxypyrimidine. The amount of 2-amino-4-chloro-6-methoxypyrimidine dissolved in DMF + water was more than that in other three systems and the ranges of values for the solubility (mole fraction) of 2-amino-4-chloro-6-methoxypyrimidine in methanol/ethanol/acetone/DMF + water were 0.0002046–0.009616, 0.0002046–0.01060, 0.0002046–0.04918 and 0.0002046–0.1197, respectively. The Jouyban-Acree (JA), Apelblat-Jouyban-Acree (AJA) and van’t Hoff-Jouyban-Acree (VJA) model were applied to calculate the solubility data of 2-amino-4-chloro-6-methoxypyrimidine in mixtures. The preferential solvation parameter δx1,3 > 0 in 0.32 < xmethanol < 1, 0.24 < xethanol < 1, 0.21 < xacetone < 1 and 0.19 < xDMF < 1, the 2-amino-4-chloro-6-methoxypyrimidine was preferentially dissolved by cosolvents and the 2-amino-4-chloro-6-methoxypyrimidine was dissolved preferentially by water within δx1,3 < 0. What’s more, the interaction between solute - solvent molecules and the internal interaction of molecules was analyzed by extend Hildebrand solubility approach (EHSA) and in the concentration range with W > δ1+2δ3, the hydrophobic groups in the 2-amino-4-chloro-6-methoxypyrimidine played a dominant role, while in other ranges self associations of solute, solvent or both were observed. The dissolution thermodynamic properties were obtained by van’t Hoff plots and the ranges of values for ΔsolHo (kJ∙mol−1)/ ΔsolGo (kJ∙mol−1)/ ΔsolSo (J∙mol−1) were 9.74 – 31.12, −17.84 – 4.96 and 33.79 – 161.89.
中文翻译:

2-氨基-4-氯-6-甲氧基嘧啶在四种二元溶剂混合物中的研究:溶解度测量、计算、优先溶剂化和扩展希尔德布兰德溶解度参数方法分析
2-氨基-4-氯-6-甲氧基嘧啶在甲醇/乙醇/丙酮/ N,N中的平衡摩尔分数溶解度-二甲基甲酰胺(DMF)加水在101.2 kPa下通过等温饱和法在278.15–323.15 K进行实验测量。低水分、高温的混合溶剂对2-氨基-4-氯-6-甲氧基嘧啶的溶解起到了支持作用。2-氨基-4-氯-6-甲氧基嘧啶在DMF+水中的溶解量高于其他三个体系,并且2-氨基-4-氯-6-甲氧基嘧啶的溶解度(摩尔分数)取值范围甲醇/乙醇/丙酮/DMF + 水中的甲氧基嘧啶分别为 0.0002046–0.009616、0.0002046–0.01060、0.0002046–0.04918 和 0.0002046–0.1197。应用 Jouyban-Acree (JA)、Apelblat-Jouyban-Acree (AJA) 和 van't Hoff-Jouyban-Acree (VJA) 模型计算混合物中 2-氨基-4-氯-6-甲氧基嘧啶的溶解度数据。δx 1,3 > 0 在0.32 < x甲醇 < 1、0.24 < x乙醇 < 1、0.21 < x丙酮 < 1和0.19 < x DMF < 1时,2-氨基-4-氯-6-甲氧基嘧啶优先溶解在δx 1,3 < 0范围内,2-氨基-4-氯-6-甲氧基嘧啶优先被水溶解。 此外,通过扩展希尔德布兰德溶解度分析了溶质-溶剂分子之间的相互作用以及分子的内部相互作用。方法 (EHSA) 且浓度范围内W > δ 1+2 δ 3,2-氨基-4-氯-6-甲氧基嘧啶中的疏水基团起主导作用,而在其他范围内观察到溶质、溶剂或两者的自缔合。通过 van't Hoff 图和 Δ sol H o (kJ∙mol −1 )/ Δ sol G o (kJ∙mol −1 )/ Δ sol S o (J∙mol -1 ) 为 9.74 – 31.12、-17.84 – 4.96 和 33.79 – 161.89。











































 京公网安备 11010802027423号
京公网安备 11010802027423号